Heavy charged particles for gastrointestinal cancers
Introduction
Radiation composed of particles heavier than electrons is called a particle beam, and particles heavier than helium are specifically called “heavy-ions”. Currently, carbon ion beams are used for heavy ion radiotherapy due to their unique physical and biological properties, affording precise dose delivery.
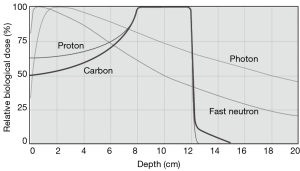
Particle radiation therapy offers unique physical advantages over photon therapy because of their defined range with a sharp high-dose Bragg peak (1-4). These unique physical advantages of particle beam warrant a superior dose distribution, which allows a more precise tumour targeting and dose escalation with better sparing of nearby organs (1) (Figure 1).
Moreover, Carbon ion has higher linear energy transfer (LET) values as compared to photons or protons which leads to its superior relative biological effectiveness (RBE) (5). Higher LET is associated with comparatively more double-strand DNA breaks, leading to irreversible cell damage independent of cell cycle or oxygenation, than other, lower-LET modalities (1). Consequently, carbon ions were chosen as the most suitable tool for cancer treatment because of its superior dose distribution and higher biological effectiveness (6).
In 1994, carbon-ion radiotherapy (CIRT) using the NIRS HIMAC, the world’s first heavy-ion accelerator complex dedicated to medical use (6), began. CIRT now enters its 24th year at NIRS. In 2003, CIRT was approved for clinical practice as a highly advanced medical technology (6). In 2016, the CIRT for bone and soft-tissue tumors received health insurance coverage. To date, over 11,000 patients have been treated with carbon irradiation at NIRS.
Two different dose delivery techniques were developed: passive beam delivery with a fixed spread-out-Bragg-Peak (SOBP), and was followed by the development of active beam scanning (4). We treated patients by the passive modulation method until 2011 and NIRS can currently treat patients with either passive scattering or three-dimensional raster scanning. Most gastrointestinal cancers, including pancreatic and colorectal cancers, are adenocarcinomas. Owing perhaps to the increased biological effect of high-LET radiation, carbon ion beams appear effective in treating traditionally radioresistant adenocarcinomas and hypoxic tumors (7,8).
With these advances, CIRT seems to be more suitable for treating selected gastrointestinal cancers than photon or proton therapy. In this review, we summarized the results of CIRT in the treatment of various gastrointestinal cancers such as locally recurrent colorectal cancer, pancreatic cancer, hepatocellular carcinoma (HCC) and esophageal cancer.
Pancreatic cancer
Pancreatic cancer is a leading cause of cancer-related death and is one of the most lethal cancers, especially in developed countries (9). The only curative treatment of pancreatic cancer is surgical resection, and even after resection, there is high rate of local and distant failure, with a 5-year survival of approximately 20% (10). Adjuvant chemotherapy after surgery is considered a widely accepted approach in resectable pancreatic cancer. However, the optimal management of borderline resectable and locally advanced unresectable pancreatic cancer is not very well defined. Sequential or concurrent chemoradiation and multiagent chemotherapy alone have been attempted in this setting, but neither have been shown to provide a significant survival advantage, and the prognosis remains poor. The relatively high rate of local recurrence following chemoradiation suggests that radiation dose escalation may offer an improved survival benefit if tolerated. Recent advances in technology, such as intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT), have shown the ability to deliver an escalated dose of radiation to the target while sparing the adjacent critical organs. However, they have failed to show any meaningful survival advantage for unresectable pancreatic cancers. This makes finding a better approach to controlling pancreatic cancer with tolerable toxicity a priority. The unique physical and enhanced radiobiological properties have prompted researchers to explore CIRT in pancreatic cancer.
CIRT in resectable pancreatic cancer
Between 2000 and 2003, a total of 22 patients with localized resectable pancreatic cancer were treated with CIRT at the National Institute of Radiological Science, Japan. Doses between 44.8 and 48 Gy RBE were delivered at 2.8–3.0 Gy RBE per fraction. The local control rate was 100% at 1 year and 87% at 2 years with an overall survival (OS) of 59% at 1 year (11).
In a phase I trial, Shinoto et al. evaluated the efficacy and tolerability of CIRT as a short-course preoperative treatment, additionally determining the maximum tolerated dose (MTD) in radiologically assessable potentially resectable pancreatic cancer patients (12). In this dose escalation study, a starting dose of 30 Gy (RBE) over 8 fractions was increased incrementally to 36.8 Gy (RBE). Between 2003 and 2010, 26 patients were enrolled. All patients completed the treatment course, and no dose-limiting toxicity (DLT) was observed. A total of 81% of patients underwent surgery 2 to 4 weeks after completing CIRT. Four patients (15%) were unable to undergo surgery because of metastatic progression, and one patient refused it. 90% of the patients underwent R0 resection. Observed progression included distant metastasis in 65%, while 8% experienced regional relapse; no local recurrence was noted. The median OS was 18.6 months, with OS at 1, 3 and 5 years of 69%, 42%, and 42%, respectively. Surgical patients did not reach median OS at time of publication, with OS rates at 1, 3 and 5 years of 81%, 52% and 52%, respectively. Though MTD was not reached, 36.8 Gy (RBE) was recommended as standard secondary to excellent local control.
Standard preoperative chemoradiation is delivered over a period of 5 to 6 weeks followed by surgery after 4 to 6 weeks. Preoperative chemoradiation may reduce local recurrence following surgery, but if the tumour does not respond well to chemoradiation, there is a risk of tumour progression during this prolonged treatment. Conversely, preoperative CIRT is delivered over a period of just 2 weeks; the likelihood of tumour progression is very low because of its excellent local control and its short duration. The authors concluded that short-course preoperative CIRT is both tolerable and feasible without untolerable morbidity in cases of resectable pancreatic cancer.
CIRT in locally advanced unresectable pancreatic cancer
The management of locally advanced pancreatic cancer (LAPC) is controversial and has been extensively discussed in the last decade. Increasing local control using radiotherapy is expected to influence the survival, but radiosensitivity of the upper abdominal organ limits dose to suboptimal levels for controlling disease. A significant proportion of LAPC patients may not benefit from extensive local treatment, as they develop distant metastasis within a few weeks. CIRT has shown promising results in LAPC.
In 2016, Shinoto et al. performed a dose-escalation trial in the setting of LAPC to determine the MTD of CIRT and gemcitabine. The secondary endpoints were late toxicities, freedom from local progression (FFLP) and the OS (13). Between 2007 and 2012, 72 patients were enrolled. CIRT was delivered in 12 fractions over 3 weeks. Initial CIRT dose was 43.2 Gy (RBE), with gemcitabine increased from 400 to 700, then to 1,000 mg/m2. Gemcitabine dose was then fixed at 1,000 mg/m2, with CIRT dose escalated to 55.2 Gy (RBE) in 5% increments. Grade 3–4 haematological toxicities were seen in 53% of patients. Dose-limiting toxicities were observed in only 3 patients: 2 patients suffered from grade 4 neutropenia with 43.2 Gy RBE, and grade 3 intratumoral infection was observed in 1 patient treated with 50.4 Gy (RBE). No grade >3 acute GI ulceration was observed; one patient treated with 50.4 Gy (RBE) experienced a grade 3 gastric ulcer with hemorrhage. The 1- and 2-year OS rates were 73% and 35%, respectively. In the high-dose group with stage III disease (>45.6 Gy RBE) the 2-year FFLP and OS were 40% and 48%, respectively, compared to 9% and 23% in the low-dose group (43.2 Gy RBE). This study did not conclude an MTD; however, they did not administer a dose greater than 55.2 Gy RBE because of the risk of severe late toxicities. A notable finding in this study was that CIRT including the primary tumour and the subclinical lymph nodal areas along with a full dose of gemcitabine was well tolerated by this cohort. This study set the platform for escalating the dose of CIRT while administering a full dose of gemcitabine, which might provide the maximal locoregional and systemic effects essential for managing this deadly disease.
Recently, Kawashiro et al. conducted a retrospective analysis of the efficacy of high-dose CIRT along with a full dose of Gemcitabine in LAPC (14). A total of 72 patients with LAPC from 2012 to 2014 at 3 institutions were included in this study. The prescribed dose of CIRT was 52.8 Gy (RBE) or 55.2 Gy (RBE), both delivered in 12 fractions, along with concurrent injection of gemcitabine at 1,000 mg/m2 on days 1, 8 and 15. Seventy-eight percent of patients received concurrent chemotherapy. OS rates were 73% at 1 year and 46% at 2 years, constituting a median OS of 21.5 months. Cumulative local recurrence at 1- and 2-years were 16% and 24%, respectively. In this study, the proportion of patients receiving concurrent chemoradiation was significantly larger in the higher dose 55.2 Gy (RBE) cohort than in the 52.8 Gy RBE cohort (P=0.004). Although no marked difference in local control was noted between the two cohorts, the trend in distant metastasis-free survival (DMFS) was better in improved in high-dose vs. low-dose patients. This suggests that concurrent chemotherapy may have some influence on the improved DMFS with 55.2 Gy (RBE). Regarding acute toxicities, 26% of patients suffered from grade 3–4 haematological toxicities related to the use of gemcitabine-based chemotherapy. Only 1 patient (1%) developed a grade 3 duodenal ulcer, a much better rate than with IMRT, SBRT or proton beam therapy, as reported in the literature (15,16). In comparison with other modalities, CIRT proved to be a safe and feasible treatment in LAPC with an excellent outcome (Table 1).
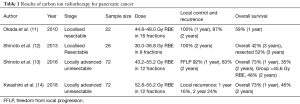
Full table
More recent data on patients with LAPC who received CIRT with concurrent chemotherapy have shown an impressive 2-year OS of 60% (data yet to be published). Kawashiro et al. conducted a dosimetric study that showed the feasibility of dose escalation yet to be confirmed in clinical trials (17). Combs et al. from Germany are currently investigating CIRT for LAPC with concurrent and adjuvant gemcitabine in a prospective setting. Furthermore, the first phase III randomised controlled trial CIPHER comparing CIRT vs. IMRT (along with chemotherapy in both arms) for LAPC will start recruiting patients soon (18,19).
Postoperative rectal cancer recurrence
With ongoing advances in chemotherapy and surgical techniques, the postoperative local recurrence rate at 5 years has fallen to between 5% and 15%. For resectable pelvic recurrent tumors, surgical resection remains the best way to achieve a cure, but in many cases, those tumors are difficult to remove completely, and high morbidity or mortality with surgery can be expected. Therefore, a large proportion of patients with local recurrence should be recommended to receive radiotherapy or chemoradiotherapy instead. Five-year survival rates for locally recurrent rectal cancer treated conventionally with chemoradiation range from 20% to 25% (20,21); even when combined with surgical resection, rates only improve to 30% to 45% (22-24).
CIRT has been used to treat locally recurrent rectal cancer from 2001 so as to improve long-term local control and survival rates. As locally recurrent tumors often develop under severe hypoxic conditions, CIRT can provide great advantages over conventional radiotherapy due to its stronger biological effect with a high LET. According to a recent publication from NIRS, covering April 2001 to August 2012 (1), for 180 patients treated with 67.2–73.6 Gy (RBE) in 16 fractions, 5-year local control was 88% with concurrent survival of 59%. No adverse events more severe than grade 3 (NCI-CTC/RTOG_EORTG) were observed, and only two cases with late grade 3 skin reactions and one case of late grade 3 gastrointestinal reaction were observed, suggesting that this approach is acceptable. Figure 2 shows example of dose distributions with CT and MRI images from before and after CIRT in one patient (Figure 2).
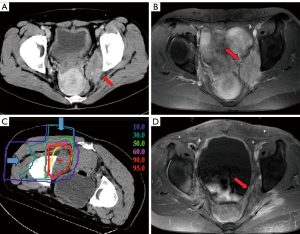
However, a significant proportion of patients failed to meet the eligibility criteria for CIRT, as the bladder or digestive tracts, including the jejunum and hypovascular colon or rectum, lay too close to the planned irradiation field. Spacers have been implanted to separate radiosensitive tissue from the irradiation field (including omentum, a PTFE sheet, or others). Use of spacer was found to be noninferior in terms of local control and survival rates to cases without a spacer, but there was a slightly increased risk of pelvic infection. A new trial to remove irradiated large and small bowel shortly after CIRT has now been designed to take place of the spacer procedure.
CIRT for previously irradiated locally recurrent rectal cancer
Standard treatment for locally advanced rectal cancer is preoperative radiotherapy or chemoradiotherapy followed by total mesorectal excision (TME). As such, a significant proportion of cases with local recurrence will have been irradiated with >50 Gy to the whole pelvis. Even for these cases, re-irradiation to the recurrent tumour can potentially improve the local control and survival. The concern is that a re-irradiation of such patients would increase the risk of acute and late toxicity to radiosensitive organs compared to cases without any history of irradiation of the pelvis. A recent publication from NIRS (5) describes the treatment of 23 locally recurrent rectal cancer patients reirradiated with 70.4 Gy (RBE) in 16 fractions. The 3-year OS rate was 65%. These results seemed superior to those from other studies concerning the combination treatment of chemotherapy and reirradiation (25-27).
A significant proportion of locally recurrent rectal cancer patients present with inoperable disease; CIRT may offer significant therapeutic opportunity for these patients, appearing both safe and effective for local disease management, including upon reirradiation, without unacceptable morbidity.
HCC and liver metastasis from colorectal cancer
HCC is conventionally treated with hepatectomy, transcatheter arterial embolization (TAE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and/or liver transplantation (6). Eligibility depends on patient status and tumor characteristics. Ineligible patients may be referred for radiotherapy. However, conventional irradiation is limited by the radiosensitivity of the liver, with significant risk of radiation-induced liver disease (5,28-31).
NIRS began CIRT for HCC in 1995. Four clinical trials were conducted for treatment dose-setting with increasing hypofractionation, beginning with 15 and eventually reducing to 4 total fractions (6,32). Following these trials, a phase II trial was performed using 52.8 Gy (RBE) in 4 fractions. From 2003, a trial of 2-fraction CIRT for patients with HCC began, and is currently the standard of treatment at NIRS. Inclusion criteria include: (I) clinically diagnosed HCC, (II) HCC treatable in a single radiation field, (III) distance between target and gastrointestinal tract within approximately 1 cm, (IV) no evidence of extrahepatic metastasis, (V) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and (VI) no uncontrollable ascites. Exclusion criteria include: (I) Child-Pugh class C hepatic disorder, (II) any history of irradiation of the lesion, and (III) major invasion of the hepatic or portal vein. Thus, patients deemed unamenable to other treatments, had recurrence following a separate treatment, or had no prospect of obtaining good effect with other existing therapies, could be enrolled. The irradiation field was established with a 3D planning system using 2.5-mm-slice CT images (6). The planning target volume (PTV) was defined as a 1- to 1.2-cm margin around the gross tumor volume (GTV) (6). Between 2003 and 2012, 133 patients with HCC were treated with 2-fraction CIRT at NIRS (6). No grade ≥4 toxicities were noted. In the higher-dose group [45.0 Gy (RBE)], local control rates in the smaller tumor (≤5 cm) and larger-tumor (>5 cm) groups were 97% and 100% at 1 year and 81% and 80% at 3 years, respectively, with overall survival of 88% and 82%, 61% and 59%, and 43% and 44% at 1, 3, and 5 years, respectively (6,33). The results of the present study seem to be comparable to the results obtained from studies in which hepatic resection was performed.
Thus, hypo-fraction CIRT, which was established at the NIRS, has shown promising results against localized HCC. By 2013, four carbon-ion facilities in Japan were treating HCC patients using hypofractionated (≤4 fractions) CIRT (34). The Japan Carbon Ion Radiation Oncology Study Group (J-CROS) recently conducted a multi-institutional evaluation of the efficacy of hypo-fractionated CIRT for HCC (34). Between 2005 and 2014, 174 patients were treated. Prescribed CIRT doses ranged from 48.0 Gy (RBE) in 2 fractions (n=46), to 52.8 Gy (RBE) (n=108) and 60.0 Gy (RBE) (n=20) in four fractions (34). Local control at 1, 2 and 3 years was 95%, 88% and 81%, respectively, while overall survival was 95%, 83% and 73%, respectively. Ten Grade 3 or 4 treatment-related toxicities were observed (5.7%), with radiation-induced liver disease noted in three (1.7%). In this study, most patients were not eligible for hepatectomy or percutaneous ablation therapy. This study suggested that the outcome of CIRT for HCC was favorable in comparison to the outcomes of other curative treatments (34).
At NIRS, a clinical trial of hypofractionated CIRT for liver metastasis from colorectal cancer was conducted (35), constituting a prospective single-arm dose escalation phase I study using single fraction CIRT beginning in 2006. Patients with evident vascular invasion or prior treatment to the lesion were not eligible. Twenty-nine patients have been enrolled and treated (35). Prescribed doses were: 36 Gy (RBE) (n=3), 40 Gy (RBE) (n=2), 44 Gy (RBE) (n=4), 46 Gy (RBE) (n=6), 48 Gy (RBE) (n=3), 53 Gy (RBE) (n=8), and 58 Gy (RBE) (n=3). 3-year OS was 78%, with local control in the higher dose (≥53 Gy (RBE)) group of 82%. No grade ≥3 acute toxicity attributable to CIRT was observed. However, two cases of late grade 3 liver toxicity secondary to biliary obstruction was noted in the 53 Gy (RBE) cohort. Single-fraction CIRT for colorectal liver metastasis appears feasible and effective, though the central hepatic portal region should be avoided (35). Hypo-fractionated CIRT is a safe, effective and promising therapeutic tool for HCC and liver metastasis from colorectal cancer.
Esophageal cancer
The unique physical and biological advantages of CIRT lead to expectations of good treatment effect for esophageal cancer patients (6) (Figure 3).
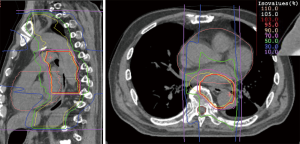
At NIRS, a phase I/II clinical trial was conducted between 2004 and 2008, involving dose-escalation of short-course preoperative CIRT for resectable esophageal cancer (36). Thirty-one patients with thoracic esophageal carcinoma were enrolled in the trial. The radiation dose was escalated from 28.8 to 36.8 Gy (RBE). Between 4 and 8 weeks following irradiation, esophagectomy and LN dissection were performed. Only one patient showed grade >3 toxicity. Postoperative acute respiratory distress syndrome was observed in one case; however, we did not consider this to be a direct effect of CIRT. Local tumor control was excellent. CIRT alone achieved pathological complete response (CR) in 12 patients (39%), and these patients demonstrated improved survival. Notably, 12 (60%) of T1 and T2 cases achieved pathological CR, suggesting that CIRT alone might be a potentially curative treatment for Stage I esophageal cancer. At the same time, this study identified some issues that need to be resolved. The risk of postoperative LN recurrence was not controlled by CIRT alone in advanced cases, such as T3 or LN-positive cases (36).
On receiving these results, we began a phase I/II CIRT monotherapy dose escalation study for T1bN0 esophageal cancer from 2007, and a phase I/II preoperative short-course CIRT combined with chemotherapy for stage II/III esophageal cancer from 2012.
Regarding CIRT for T1bN0 esophageal cancer, a 12 fraction/3 week 43.2 to 50.4 Gy (RBE) dose regimen was employed. 38 patients have been treated. Grade 3 acute esophagitis and Grade 3 acute hematotoxicity were observed in 4 and 3 cases, respectively. However, late Grade ≥3 toxicities only occurred in one case. The patient in question developed aspiration pneumonitis; however, this did not seem to be a direct effect of CIRT.
Thirty-two cases (84.2%) showed a complete response after treatment. Local recurrence occurred in 11 patients and salvage surgery or endoscopic resection were performed in all 11 patients (surgery: n=4, ER: n=7). With a median follow-up of 43 months, the 3- and 5-year OS was 86% and 81%, respectively. However, there were 13 patients in high-risk surgery and chemoradiotherapy groups because of their complications and ages. The 3- and 5-year cause-specific survival was 97% and 91%, respectively. According to The Registration Committee for Esophageal Cancer of the Japan Esophageal Society, the 3- and 5-year survival of stage I patients who underwent esophagectomy was 85.0% and 76.8%, respectively (37). CIRT may be both safe and effective for stage I (T1bN0) esophageal cancer, and may constitute an alternative to surgery or chemoradiotherapy.
The phase I/II study of preoperative short-course CIRT combined with chemotherapy for stage II/III esophageal cancer began in 2012, and is ongoing. Patients with stage II/III resectable esophageal cancer (except for those with T4 disease) were eligible for inclusion. Dose was escalated from 33.6 Gy (RBE) in 5% increments only when no severe adverse events were observed. To date, 19 cases have been treated with this protocol and the irradiation dose is now 36.8 Gy (RBE). Although 13 of the 19 cases involved had Stage III disease and 8 of the 19 cases involved T3 disease, more than half the cases achieved a pathological CR. The results suggested that the treatment might achieve strong tumor control and be highly effective in comparison to existing preoperative therapy for Stage II/III esophageal cancer. However, as the study is still ongoing, we will watch future developments.
Thus, CIRT might be a promising therapeutic option for esophageal cancer, yet CIRT for esophageal cancer requires further exploration.
Conclusions
CIRT has demonstrated promising potential for delivering sufficiently tumoricidal effect with a minimal dose to normal tissues and is effective against locally advanced non-squamous tumors such as adenocarcinomas of gastrointestinal tract. The record of outcomes thus far has suggested the superiority of carbon ion beams over other types of irradiation in the treatment of gastrointestinal cancer. More clinical trials are needed worldwide.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Yamada S, Kamada T, Ebner DK, et al. Carbon-Ion Radiation Therapy for Pelvic Recurrence of Rectal Cancer. Int J Radiat Oncol Biol Phys 2016;96:93-101. [Crossref] [PubMed]
- Blakely EA., Ngo F, Curtis SB, et al. Heavy-ion radiobiology: Cellular studies. Adv Radiat Biol 1984;11:295-389. [Crossref]
- Hall EJ, Giaccia AJ. Radiobiology for Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93-100. [Crossref] [PubMed]
- Shinoto M, Ebner DK, Yamada S. Particle Radiation Therapy for Gastrointestinal Cancers. Curr Oncol Rep 2016;18:17. [Crossref] [PubMed]
- Tsujii H, Kamada T. Shirai T, et al. editors. Carbon-Ion Radiotherapy. Principle, Practice, and Treatment Planning. Tokyo: Springer, 2014.
- Wendling P, Manz R, Thews G, et al. Heterogeneous oxygenation of rectal carcinomas in humans: A critical parameter of preoperative irradiation? Adv Exp Med Biol 1984;180:293-300. [Crossref] [PubMed]
- Eising E, Pötter R, Haverkamp U, et al. Neutron therapy (dT, 14 Mev) for recurrence of rectal cancer: Interim analysis from Münster. Strahlenther Onkol 1990;166:90-4. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993;165:68-72. [Crossref] [PubMed]
- Okada T, Kamada T, Tsuji H, et al. Carbon ion radiotherapy: clinical experiences at National Institute of Radiological Science (NIRS). J Radiat Res 2010;51:355-64. [Crossref] [PubMed]
- Shinoto M, Yamada S, Yasuda S, et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer 2013;119:45-51. [Crossref] [PubMed]
- Shinoto M, Yamada S, Terashima K, et al. Carbon Ion Radiation Therapy With Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2016;95:498-504. [Crossref] [PubMed]
- Kawashiro S, Yamada S, Okamoto M, et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int J Radiat Oncol Biol Phys 2018;101:1212-21. [Crossref] [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A Phase I/II Trial of Intensity Modulated Radiation (IMRT) Dose Escalation with Concurrent Fixed-Dose Rate Gemcitabine (FDR-G) in Patients with Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 Multi-Institutional Trial Evaluating Gemcitabine and Stereotactic Body Radiotherapy for Patients with Locally Advanced Unresectable Pancreatic Adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Kawashiro S, Mori S, Yamada S, et al. Dose escalation study with respiratory-gated carbon-ion scanning radiotherapy using a simultaneous integrated boost for pancreatic cancer: simulation with four-dimensional computed tomography. Br J Radiol 2017;90:20160790. [Crossref] [PubMed]
- Combs SE, Habermehl D, Kieser M, et al. Phase I Study Evaluating the Treatment of Patients with Locally Advanced Pancreatic Cancer with Carbon Ion Radiotherapy: The PHOENIX-01 trial. BMC Cancer 2013;13:419. [Crossref] [PubMed]
- Mohamad O, Yamada S, Durante M. Clinical Indications for Carbon Ion Radiotherapy. Clin Oncol (R Coll Radiol) 2018;30:317-29. [Crossref] [PubMed]
- Tanaka H, Yamaguchi T, Hachiya K, et al. Radiotherapy for locally recurrent rectal cancer treated with surgery alone as the initial treatment. Radiat Oncol J 2017;35:71-7. [Crossref] [PubMed]
- Kim MS, Choi C, Yoo S, et al. Stereotactic body radiation therapy in patients with pelvic recurrence from rectal carcinoma. Jpn J Clin Oncol 2008;38:695-700. [Crossref] [PubMed]
- Nielsen M, Rasmussen P, Pedersen B, et al. Early and Late Outcomes of Surgery for Locally Recurrent Rectal Cancer: A Prospective 10-Year Study in the Total Mesorectal Excision Era. Ann Surg Oncol 2015;22:2677-84. [Crossref] [PubMed]
- Harris CA, Solomon MJ, Heriot AG, et al. The Outcomes and Patterns of Treatment Failure After Surgery for Locally Recurrent Rectal Cancer. Ann Surg 2016;264:323-9. [Crossref] [PubMed]
- You YN, Skibber JM, Hu CY, et al. Impact of multimodal therapy in locally recurrent rectal cancer. Br J Surg 2016;103:753-62. [Crossref] [PubMed]
- Das P, Delclos ME, Skibber JM, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys 2010;77:60-5. [Crossref] [PubMed]
- Sun DS, Zhang JD, Li L, et al. Accelerated hyperfractionation field-involved re-irradiation combined with concurrent capecitabine chemotherapy for locally recurrent and irresectable rectal cancer. Br J Radiol 2012;85:259-64. [Crossref] [PubMed]
- Koom WS, Choi Y, Shim SJ, et al. Reirradiation to the pelvis for recurrent rectal cancer. J Surg Oncol 2012;105:637-42. [Crossref] [PubMed]
- Hansen E, Roach M. editors. III. Handbook of Evidence-Based Radiation Oncology. New York, NY: Springer Science & Business Media, 2010.
- Kanai T, Furusawa Y, Fukutsu K, et al. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res 1997;147:78-85. [Crossref] [PubMed]
- Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201-10. [Crossref] [PubMed]
- Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys 2004;59:1468-76. [Crossref] [PubMed]
- Kasuya G, Kato H, Yasuda S. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer 2017;123:3955-3965. [Crossref] [PubMed]
- Kudo M, Arii S, Ikari I, et al. Report of the 18th nationwide follow-up surver of primary liver cancer in Japan. Kanzo 2010;51:460-84. [Crossref]
- Shibuya K, Ohno T, Terashima K. Short-course carbon-ion radiotherapy for hepatocellular carcinoma: A multi-institutional retrospective study. Liver Int 2018;38:2239-47. [Crossref] [PubMed]
- Makishima H, Yasuda S, Isozaki Y, et al. Single fraction carbon ion radiotherapy for colorectal cancer liver metastasis: A dose escalation study. Cancer Sci 2019;110:303-9. [PubMed]
- Akutsu Y, Yasuda S, Nagata M, et al. A phase I/II clinical trial of preoperative short-course carbon-ion radiotherapy for patients with squamous cell carcinoma of the esophagus. J Surg Oncol 2012;105:750-5. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive Registry of Esophageal Cancer in Japan, 2011. Esophagus 2018;15:127-52. [Crossref] [PubMed]