
Neoadjuvant therapy and pancreatic cancer: a national cancer database analysis
Introduction
Pancreatic cancer is the 4th leading cause of cancer death in the United States with 55,440 new diagnoses and 44,330 deaths estimated for 2018 (1). Survival continues to remain poor with the 5-year survival in patients undergoing an R0 resection is 25% (2-4). At diagnosis, approximately 26% of pancreatic cancer is deemed resectable, and at the time of operation, it has been reported that 28% of resectable patients will actually have R1 resection after histological examination (5,6) and approximately 38% of patients will have recurrence most commonly as distant metastases suggesting possible unidentified micrometastasis (7,8). The current recommendation by the National Comprehensive Cancer Network (NCCN) for resectable and borderline resectable pancreatic cancer is to do definitive surgery followed by adjuvant therapy (9). Adjuvant therapy has been shown to increase 5-year survival even further to 28–37% (3,4,10). Unfortunately, not all resected patients will end up receiving adjuvant therapy due to postoperative complications (11,12).
Neoadjuvant therapy has the potential to improve R0 resection, allow early therapy for micrometastases. In addition, this will potentially allow metastatic pancreatic cancer to declare itself and improve patient selection for improved surgical outcomes. Studies have already demonstrated the safety of neoadjuvant therapy in resectable and borderline resectable pancreatic cancer with no increase in short-term post-operative complications (13,14). The addition of neoadjuvant therapy has been shown to improve survival in borderline resectable and locally advanced pancreatic cancer but there is minimal evidence to suggest its use in resectable pancreatic cancer (15-17). Our study’s purpose is to demonstrate the utilization of neoadjuvant CRT and CT as beneficial for patients diagnosed with resectable pancreatic cancer with the goal of improving overall survival (OS).
Methods
Patients
The National Cancer Database (NCDB) is a dataset maintained by the American College of Surgeons and the American Cancer Society and collects patient data from >1,500 centers across the United States. Our patient population was obtained from the Pancreatic Participant Use Data File (PUF). Data represents more than 70% of newly diagnosed cancer cases nationwide. PUF’s are entirely de-identified data files available to selected investigators at CoC-approved institutions for the advancement of patient care. After obtaining approval from the Sarasota Memorial Hospital institutional review board, we queried the NCDB for patients with a diagnosis of pancreatic adenocarcinoma who underwent surgery between 2004 and 2013. Patients were stratified as: upfront (UFS), neoadjuvant chemotherapy only (NCT), or neoadjuvant chemoradiation (NCRT).
Statistics
Baseline univariate comparisons of patient characteristics between the upfront surgery (UFS) patients, NCT patients, and chemoradiation patients were made for continuous variables using the Mann-Whitney U and Kruskal Wallis tests as appropriate. Pearson’s Chi-square test and Fisher’s exact test was used to compare categorical variables when appropriate. OS was defined from the time of diagnosis to death or last contact. Survival time was censored for patients alive at the end of the study period. Kaplan-Meier survival analysis was used to generate OS curves and estimate median survival with 95% CIs for each group. Survival distributions were compared across groups using the log-rank test.
Multivariable Cox proportional hazard models were developed comparing treatment methods (UFS, NCT, neoadjuvant chemoradiation). Predictors of long-term survival included in the models were age, sex, pathologic T-stage, pathologic N-stage, tumor grade, tumor size, lymph nodes harvested, number of lymph of positive lymph nodes, surgical margins, institution volume, adjuvant therapy and use of induction therapy. Facility volume was calculated as the total number of cases within a facility for a given year.
To correct for baseline differences among treatment groups, propensity score matching (PSM) was used to match for age, tumor size, and facility volume. Matching occurred on a 1:1 basis and only exact matches were allowed. PSM creates treatment groups in a way that approximates the effect of randomization, and therefore partially removes the bias that typically accompanies treatment assignment in nonrandomized studies. All statistical tests were two-sided and α (type I) error <0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 23.0 (IBM®, Chicago, IL, USA). This study was approved as exempt by the Institutional Review Board.
Results
We identified 26,653 patients from the NCDB who underwent resection for pancreatic cancer of which 1,204 (4.5%) underwent NCT, 1,482 (5.6%) underwent neoadjuvant chemoradiation (NCRT) and 23,877 (90%) underwent UFS (Table 1). Significant differences were noted for age, Charlson-Deyo index, tumor size, lymph nodes removed, lymph nodes positive, pathologic T and N stage, grade, 30 and 90-day mortality, surgical margins, facility volume, and adjuvant therapy. The complete response rates were 1.7% for NCT and 3.1% for NCRT (P<0.001). We used propensity score matched (PSM) analysis of neoadjuvant therapy (NCT and NCRT) versus UFS matched by age, tumor length, and facility volume. After PSM, 3,066 patients were identified with significant differences in lymph nodes removed, lymph nodes positive, pathologic T and N stage, 30-day mortality, surgical margins, and adjuvant therapy. No adjuvant therapy was given in 62% of patients treated with neoadjuvant therapy compared to 28% for UFS patients (P<0.001).
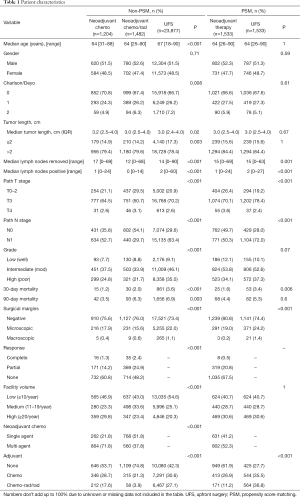
Full table
R0 resection was statistically improved in both NCT and NCRT versus UFS (Table 2). In patients treated with single agent chemotherapy, only NCRT had significantly higher R0 rates compared UFS (P=0.02). SA-NCT did not improve R0 rates (P=0.26). There was no difference in R0 between NCT and NCRT. Patients treated with multiagent chemotherapy had higher R0 rates compared to patients treated with single agent chemotherapy. There were improved R0 resection rates associated with NCT and NCRT (P<0.001) compared to UFS. There was no difference in R0 between NCT and NCRT.
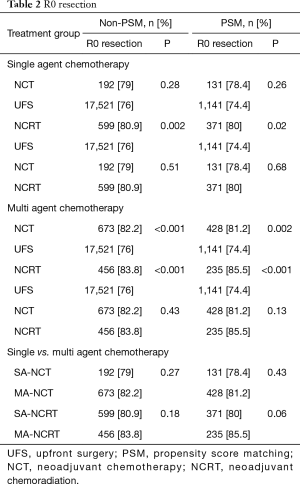
Full table
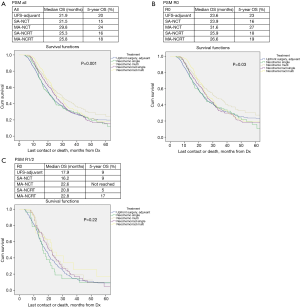
OS in patients who received NCT and NCRT was compared to UFS who received adjuvant therapy. After PSM, the median OS for UFS, SA-NCT, MA-NCT, SA-NCRT, and MA-NCRT was 21.9, 21.5, 29.8, 25.3, and 25.8 months in all patients (P=0.001) (Figure 1A), and 23.6, 23.9, 31.6, 25.9, and 26.6 months in R0 patients (P=0.03), respectively (Figure 1B). There was no difference in OS in patients with R1/2 resection (Figure 1C).
Univariate analysis of the PSM group revealed that increasing age, Charlson-Deyo index, pathologic T and N stage, higher grade, tumor size, and positive surgical margins were associated with increased mortality. MA-NCT and MA-NCRT were associated with decreased mortality, while gender, SA-NCT, SA-NCRT, and facility volumes were not prognostic (Table 3).
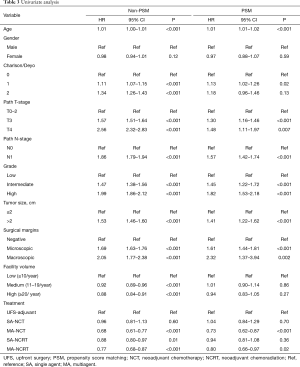
Full table
Multivariate analysis of the PSM group revealed that increasing age, Charlson-Deyo index, pathologic N1, higher grade, tumor size >2 cm, and positive surgical margins were associated with increased of mortality. MA-NCT was the only factor associated with decreased mortality, while SA-NCT, SA-NCRT, MA-NCRT, gender, pathologic T-stage, and facility volumes were not prognostic (Table 4).
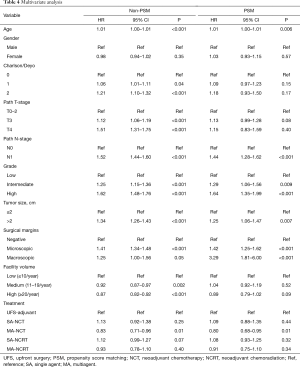
Full table
Discussion
This study represents one of the largest retrospective reviews on neoadjuvant therapy for pancreatic cancer. There was improved OS associated with MA-NCT in pancreatic cancer patients compared to UFS with adjuvant therapy. While there was improved survival with MA-NCRT on UVA, it did not hold up on MVA. In addition, while MA-NCRT had the highest R0 resection rates, there was not a statistically significant difference compared to MA-NCT. SA-NCT did not affect OS or R0 rates. While there was improved OS in high volume centers, this did not hold up after PSM.
Neoadjuvant therapy in pancreatic cancer continues to be a topic of controversy. Its use in borderline resectable cancer has been studied extensively, showing higher likelihood of achieving R0 margins and improved OS, which makes neoadjuvant therapy acceptable treatment in borderline resectable disease (6,15,18-20). Studies have previously shown that achieving R0 versus R1 resection gives a patient up to 6 months longer median survival (21). Neoadjuvant therapy in resectable pancreatic cancer also proves to have a higher R0 resection rate. Some prospective studies have shown up to 100% R0 resection rates with the use of neoadjuvant therapy in resectable pancreatic cancer (17,22). Although the previous prospective studies have small sample sizes, our outcomes did reflect an improved R0 resection rate which, by multivariate analysis, is statistically significant for survival. Neoadjuvant therapy should be considered for downstaging and improved R0 resection.
Multi-modality therapy has been established as the most effective strategy against pancreatic cancer. As of April 2017, the NCCN still recommends UFS in all resectable and borderline resectable pancreatic cancer followed by adjuvant therapy (9). Previous studies support the use of adjuvant therapy and have shown improved OS. However, many of these studies had selection bias by excluding patients who did not end up receiving adjuvant therapy (up to 60% in some cases) due to post-operative complications (3,4,23,24). If the patients who were unable to receive adjuvant therapy were included, their outcomes would likely have been poorer. In 2014, Tzeng et al. studied 167 patients, 115 who underwent neoadjuvant therapy and 52 who underwent UFS and adjuvant therapy. They discovered that 83% of the neoadjuvant therapy group completed all multimodality therapy, whereas only 58% of the UFS group was able to complete adjuvant therapy (25). The utilization of neoadjuvant therapy can allow patients to receive all necessary multimodality therapy despite surgical complications.
One argument against neoadjuvant treatment is that it allows cancer to become unresectable during a key window of opportunity for resectability. A study by Christians et al. on neoadjuvant therapy in 69 patients found that 13% of patients had progression of disease during neoadjuvant treatment. However, 100% of the disease progression was metastatic (15). Another study found that up to 76% of recurrence after surgical resection of pancreatic cancer is found to be metastatic, not local (26). These patients with progression of disease likely had occult metastases which was missed upon initial screening and allowing the disease to manifest itself may prevent an unnecessary surgery and its associated morbidity. Neoadjuvant therapy allows prompt treatment for micrometastases while giving pancreatic cancer time to present its resectability status.
Above all, we have demonstrated that neoadjuvant therapy increases median and OS. Evans et al. did a study in 2008 on 86 patients with stage I and II pancreatic cancer and showed a median survival of 34 months in patients who received neoadjuvant therapy followed by surgical resection (27). Multiple small prospective trials have shown median OS as 30–32 months in patients with resectable pancreatic cancer who underwent neoadjuvant therapy (28-30). In an NCDB analysis (1998 to 2002), a comparison of 277 patients who received preoperative radiation against 5,414 patients treated with postoperative radiation revealed no difference in OS (med OS 18 vs. 19 months) despite significantly higher number of negative margins and lymph nodes in the preoperative group (31). This finding is consistent with this study. A study from the Moffitt Cancer Center analyzed outcomes of pancreatic cancer patients who underwent UFS with adjuvant therapy (192 patients) or neoadjuvant multiagent chemotherapy followed by stereotactic radiation (61 patients) (17). In the neoadjuvant group, there was significantly higher T-stage, N-stage, and need for vascular resection and repair. R1 resections was lower after neoadjuvant therapy (3.3% vs. 16.2%, P=0.006). Postoperative morbidities and mortality were similar. Median OS favor neoadjuvant therapy (33.5 vs. 23.1 months; P=0.57). Finally, an MD Anderson study showed similar results with increased OS associated with neoadjuvant therapy with multiagent chemotherapy and chemoradiation compared to UFS (median OS 33.5 vs. 26.5 months; P=0.04) (32).
A weakness in our study includes inherent selection bias due to the study being a retrospective analysis. To counteract this bias, we included PSM. There are also limitations on the methods by which different institutions input data and a lack of data on what criteria and guidelines each institution followed for collecting data and making diagnoses. The data also lacked endoscopic ultrasound staging making it impossible to truly rule out borderline resectable pancreatic cancers. Future studies should improve upon these limitations and explore the most effective neoadjuvant therapy for all stages of pancreatic cancer.
Conclusions
Our study illustrates that neoadjuvant therapy is an effective treatment for pancreatic cancer patients diagnosed with resectable disease. Neoadjuvant therapy has potential to downstage pancreatic tumors which improves R0 resection, it ensures all patients receive multimodality therapy and most importantly, neoadjuvant therapy improves OS in patients with pancreatic cancer. Clinical trials are needed to address the role of neoadjuvant therapy in borderline and upfront resectable pancreatic cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Sarasota Memorial Hospital institutional review board and written informed consent was waived.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58. [Crossref] [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [Crossref] [PubMed]
- Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. [Crossref] [PubMed]
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg 2013;257:731-6. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Aoyama T, Murakawa M, Katayama Y, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res 2015;35:2401-9. [PubMed]
- Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012;18:118-21. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer 2007;110:1227-34. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372-7. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Cooper AB, Parmar AD, Riall TS, et al. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg 2015;19:80-6; discussion 86-7. [Crossref] [PubMed]
- Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266-74. [Crossref] [PubMed]
- Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692-700. [Crossref] [PubMed]
- Mellon EA, Strom TJ, Hoffe SE, et al. Favorable perioperative outcomes after resection of borderline resectable pancreatic cancer treated with neoadjuvant stereotactic radiation and chemotherapy compared with upfront pancreatectomy for resectable cancer. J Gastrointest Oncol 2016;7:547-55. [Crossref] [PubMed]
- Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201-19. [Crossref] [PubMed]
- Huang X, Knoble JL, Zeng M, et al. Neoadjuvant Gemcitabine Chemotherapy followed by Concurrent IMRT Simultaneous Boost Achieves High R0 Resection in Borderline Resectable Pancreatic Cancer Patients. PLoS One 2016;11:e0166606. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52-60. [Crossref] [PubMed]
- Takahashi H, Ohigashi H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 2013;258:1040-50. [Crossref] [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [Crossref] [PubMed]
- Aloia TA, Aloia TE, Lee JE, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007;204:347-55. [Crossref] [PubMed]
- Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg 2014;18:16-24; discussion 25. [Crossref] [PubMed]
- Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012;147:753-60. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 2013;119:277-84. [Crossref] [PubMed]
- Lind PA, Isaksson B, Almström M, et al. Efficacy of preoperative radiochemotherapy in patients with locally advanced pancreatic carcinoma. Acta Oncol 2008;47:413-20. [Crossref] [PubMed]
- Turrini O, Ychou M, Moureau-Zabotto L, et al. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: New neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol 2010;36:987-92. [Crossref] [PubMed]
- Colbert LE, Hall WA, Nickleach D, et al. Chemoradiation therapy sequencing for resected pancreatic adenocarcinoma in the National Cancer Data Base. Cancer 2014;120:499-506. [Crossref] [PubMed]
- Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:268-77. [Crossref] [PubMed]


