
A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: a pilot study
Introduction
Hepatocellular carcinoma (HCC) is the commonest primary cancer of the liver representing the fifth and the eighth largest cause of cancer in men and women, respectively. Further, it is considered the third most lethal cancer worldwide with about 700,000 deaths reported annually (1). Surgical resection offers a limited treatment option for only about 20% of HCC patients because of several factors, such as tumor size, multifocality and vascular invasion with a recurrence rate of post-surgery as high as 50% (2). In the same context, liver transplantation is another option offered only to early-stage HCC patients. However, the lack of donor organs makes the number of eligible patients small. Moreover, the prompt and frequent reappearance of HCC in the transplanted organ results in severely limited effectiveness (3,4). Many alternative approaches have limited usefulness and are applicable to patients with localized liver tumors only (5-7).
Using natural agents in cancer treatment is promising because of their minimal toxicity to humans compared to conventional chemotherapies and their ability to target numerous signaling pathways which is beneficial because malignant transformation and progression are multistage processes caused by gene alterations in more than one signaling pathway (8).
Turmeric, the rhizome of Curcuma longa, is widely used as a spice, coloring agent, and as a medicine for a variety of ailments. Curcuminoids are polyphenolic compounds that represent the primary chemical constituents in turmeric with the most important and abundant curcuminoids is curcumin (diferuloylmethane) (9). It has been reported that curcumin exhibits an anticancer activity against different cancer types including skin, lung, gastrointestinal, hepatic, and hematologic malignancies primarily via modulating the expression of oncogenes and tumor suppressor genes, inducing cell cycle arrest and apoptosis, and inhibiting angiogenesis and metastasis (10,11). In a prior phase I clinical trial, curcumin was found to be well tolerated at a dose as high as 8 g/day for 3 months (12). However, the efficacy of curcumin in vitro was better than that in vivo which indicated a poor bioavailability (13,14).
Piperine, an alkaloid present in black pepper (Piper nigrum) and long pepper (Piper longum), is known to enhance the bioavailability of a number of therapeutic agents including natural products (15-17). It has been reported that the co-administration of piperine with curcumin enhanced the bioavailability of the latter in rats by 154% and in humans by 2000% probably by reducing the metabolic breakdown rate, increasing the residence time in the intestine, altering the membrane lipid dynamics and changing the conformation of enzymes in the intestine (18-20).
Taurine, 2-aminoethanesulfonic acid, is an amino acid derivative that has the ability to scavenge oxygen free radicals with antitumor properties (21,22). Moreover, the addition of a certain amount of taurine to the drinking water of mice with transplanted tumors resulted in a decrease in the rate of tumor growth by 42.26%, with the extension of the mean lifespan (23).
It has been shown that interleukin-10 (IL-10), a pleiotropic cytokine produced by macrophages, T-helper 2 (Th2) cells, and B-lymphocytes, inhibits various immune reactions (24). This immunosuppressive effect may cripple macrophage activation and interferon-gamma production which are two potential mediators of antitumor response, and thus plays a major role in the development of neoplastic processes helping tumor cells to escape from host immune surveillance and potentiate them to metastasize (25). In HCV-infected, cirrhotic, and HCC patients, serum IL-10 concentration was found to be elevated. Further, its levels were associated with disease progression indicating that IL-10 may reflect the degree of inflammation in the liver and may be related to the development of HCC (26). In HCC patients, the high serum level of IL-10 may be attributed to its secretion by tumor cells, in addition to the production at the site of inflammatory changes with activated infiltrating mononuclear cells in the liver (27).
MicroRNAs (miRNAs) are short (19–22 nucleotides), endogenous and non-coding RNAs that play important regulatory functions in basic cellular processes by regulating gene expression at the posttranscriptional level. Additionally, they may act as tumor suppressors or oncogenes (28). Accumulating evidence suggests that miR-141 could function as a tumor suppressor in HCC by inhibiting the migration and invasion of tumor cells (29-31). On the other hand, miR-21 was found to be highly over-expressed in HCC where it could modulate the expression of gene products involved in the phenotypic characteristics of cancer cells such as cell growth, migration, and invasion and thus may contribute to tumor growth and spread (32-34).
Based on the existing evidence, this study was conducted to evaluate the effect of a natural agents’ combination treatment, presented by curcumin, piperine, and taurine, on serum IL-10 level and the expression level of circulating miR-141 and miR-21 in patients with HCC.
Methods
Study population
This study is a part of a prospective, single center, single arm, open-label phase II trial in which patients with HCC were recruited from the medical oncology clinic at the National Cancer Institute (NCI), Cairo University in the period from November 2015 to March 2017.
Patients, who failed standard therapeutic approaches and had no other effective treatment option, were eligible if they were ≥18 years old with unresectable locally advanced or metastatic HCC following Barcelona Clinic Liver Cancer (BCLC) classification confirmed by pathological diagnosis or standard clinical and radiologic criteria. The disease should not be amenable to percutaneous ablation or transarterial therapy. Patients’ functional status had to be Child-Pugh class A or B and must have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Further eligibility criteria included adequate organ function defined by alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities <5 folds above the upper limit of normal (ULN), total bilirubin level <3 mg/dL, creatinine level <2 folds above ULN, hemoglobin level ≥9 g/dL, absolute neutrophil count ≥1,500/µL and platelet count ≥75,000/µL.
Exclusion criteria were as follows: patients fitting any modality of standard active management, Child-Pugh class C, performance status >2, HIV infection, significant medical co-morbidities, chronic use of systemic steroids or immunosuppressive therapy, brain metastases, active bleeding, pregnancy or lactation, impairment of gastrointestinal function or gastrointestinal disease.
Patients previously treated with systemic chemotherapy could be enrolled after a washout period of at least 4 weeks.
Study design
Twenty patients, who fulfilled all the eligibility and exclusion criteria, took a 500 mg of taurine (Solgar, NJ, USA) once daily and eight capsules divided in a thrice-daily regimen, each contained 500 mg of curcuminoids (70–80% curcumin, 15–25% demethoxycurcumin and 2.5–6.5% bisdemethoxycurcumin) and 5 mg of piperine as a bioavailability enhancer (Sabinsa Corp., NJ, USA), orally after food for three successive cycles; each was a 30-day cycle. Patients received the best available supportive care during the study time and were followed-up for a total period of 24 months or until death, whichever occurred first.
Evaluation during the study
The primary endpoint in this study was the analysis of the combined treatment effect on serum IL-10 and miRNA expression levels. Therefore, their levels were measured prior to the administration of the treatment, and after each treatment cycle. Further, a complete history, physical examination, blood tests including a CBC with differential, renal and liver function tests, and alpha-L-fucosidase (AFU), were obtained in all patients at baseline, repeated every 30 days prior to the administration of the subsequent cycle, and after the last cycle.
IL-10 level
Serum level of IL-10 was measured by ELISA kit (Cat No. 950.060.096) according to the manufacturer’s instruction (Diaclone Research, Besancon, France). The sensitivity of the assay was 5 pg/mL with an intra-, and inter-assay coefficients of variation (CV) of 3.5% and 7.5%, respectively. The test was performed on ChroMate® microplate reader (Awareness Technology Inc., FL, USA).
miRNAs expression
Total RNA including miRNAs and other small non-coding RNAs >18 nucleotides were extracted from 100 µL of serum using miRNeasy serum/plasma kit (Qiagen) according to the protocol provided by the manufacturer. One µg of the total RNA was reverse transcribed to synthesize first cDNA strand using miScript II RT Kit (Qiagen). Specific miScript primer assays for miR-141, and -21 (Cat No. MS00008680, and MS00009079, respectively) along with the universal upstream primers contained in the miScript SYBR® green PCR kit (Qiagen) were used for real-time reactions in a final volume of 25 µL with 2.5 µL of cDNA as a template, 2.5 µL of 10× miScript universal primer, 2.5 µL of 10× miScript primer assay, and 12.5 µL of 2× QuantiTect SYBR® green PCR master mix. PCR conditions were as follows: 10 min at 95 °C for initial denaturation, followed by 40 amplification cycles of denaturation at 95 °C for 15 sec, 30 sec at 55 °C for annealing, and 30 sec at 70 °C for extension. PCR reactions were carried out in MicroAmp® fast optical 96-well reaction plate with MicroAmp® optical adhesive film (Applied Biosystems) The plate was loaded into the 7500 Real-Time PCR system (Applied Biosystems, CA, USA). Each analysis was done in triplicate; the melt curve analyses of all real-time PCR products were performed and shown to produce a single DNA duplex. Quantitative measurements were determined after each treatment cycle using the 2-ΔΔCt method (35) and the baseline expression was considered as a control. The expression of mature miRNAs was normalized to human RNU68 snRNA (Cat No. MS00033712) purchased from Qiagen (Hilden, Germany). The baseline expression was calculated using the mean ΔCt values of ten age- and sex-matched healthy subjects.
Statistical analysis
Analyses were performed using SPSS version 20 (IBM Corp., NY, USA). The normality of data distribution was tested with the Shapiro-Wilk test; normally distributed data are expressed as mean ± SD, non-normally distributed data are expressed as median and interquartile range (25th and 75th percentile), and categorical variables are expressed as frequencies (percentages). Continuous variables were compared using ANOVA for repeated measures followed by Bonferroni correction for adjustment of multiple comparisons or Friedman’s test followed by Dunn post hoc for multiple comparisons as appropriate. Overall survival (OS) with its corresponding 95% confidence interval (CI) was measured from the day of the first administration of the treatment till the date of death or the last follow up. The samples were divided into two groups (high vs. low) by a cutoff value which was determined as the median value for IL-10 and as the median value of the -ΔΔCt for miRNAs at the baseline level and the log-rank test was performed to assess the association between IL-10 and miRNAs levels with the time to survival along with Cox’s proportional hazards models to estimate the hazard ratio (HR). Kaplan-Meier curves were used to visually examine the cumulative probability of remaining alive for IL-10 and miRNAs sub-groups. All P values were 2-sided and a P value <0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
The patient baseline characteristics are described in Table 1. The mean patients’ age was 58.95±5.47 years; most of them (75%) had BCLC stage B disease and 60% were with ECOG performance status of 0. Moreover, 60% of patients had a tumor size >5 cm with median serum levels of 610 U/L for AFU and 37.92 pg/mL for IL-10 at the time of study entry.
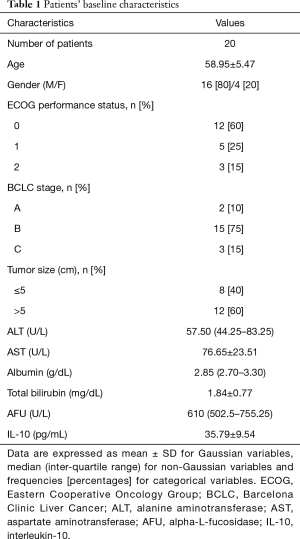
Full table
Variation of serum levels of some biochemical parameter before, during, and after the treatment
Table 2 shows that among the biochemical parameters, activities of ALT and AST in addition to total bilirubin and AFU levels in sera of HCC patients turned out to be decreased with the treatment relative to the baseline levels. Multiple comparisons showed that serum activities of ALT and AST and the level of AFU were significantly decreased after the first treatment cycle compared to those at the baseline (P=0.004, P=0.005, and P=0.040, respectively). Further, serum activities of ALT and AST and the levels of total bilirubin and AFU were significantly decreased after 60 days of treatment compared to the baseline levels (P=0.004, P<0.001, P=0.002, and P<0.001, respectively). On the other hand, total bilirubin level and the activities of ALT and AST were significantly decreased compared to those after one cycle of treatment (P=0.025, P=0.003, and P<0.001, respectively). After 3 cycles of treatment, serum activity of AST and the levels of total bilirubin and AFU showed a significant decrease compared to the baseline levels (P<0.001, P=0.001 and P<0.001, respectively), to those after one cycle of treatment (P=0.001, P<0.001, and P<0.001, respectively), and to those after two cycles of treatment (P=0.013, P=0.001, and P=0.019, respectively). Serum activity of ALT was significantly decreased compared only to the baseline level (P=0.001), and to that after one cycle of treatment (P=0.002).
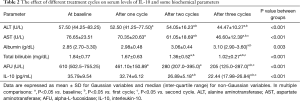
Full table
Meanwhile, albumin level was significantly increased in sera of HCC patients only after three cycles of treatment in comparison with that at the baseline (P<0.001), and with that after 30 days of treatment (P=0.032).
Effect of treatment on serum IL-10 level
The combined treatment, composed of curcumin, piperine, and taurine, resulted in a significant decrease in the serum level of IL-10 after two cycles of treatment compared to the baseline level (P<0.001), and to that after 30 days of treatment (P=0.001). Additionally, serum IL-10 level after three treatment cycles showed a significant decrease in comparison with that at the baseline, as well as after one, and 2 cycles of treatment (P<0.001, P<0.001, and P=0.005, respectively) (Table 2).
Change of circulating miRNAs expression level in response to the treatment
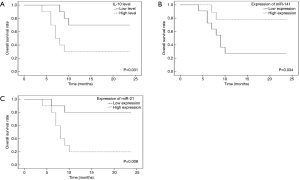
With respect to miR-141, a non-significant up-regulation of its expression was observed after the second and the third treatment cycles either compared to the expression after the first cycle or compared to each other (P=0.549) (Figure 1A).

On the contrary, the expression of miR-21 was affected by the treatment showing a significant down-regulation between cycles (P<0.001). The expression was down-regulated by 4 folds after the second cycle and by 70 folds after the third cycle of treatment compared to the expression after the first cycle (P=0.006, and P<0.001, respectively). Further, the expression was down-regulated by 17 folds after the third cycle of treatment compared to that after the second one (P=0.006) (Figure 1B).
Association of IL-10 and miRNAs pre-treatment levels with the OS in HCC patients
All patients received the three cycles of treatment; 50% of patients were alive after one year with a median OS of 17.00 months (95% CI: 11.67–19.73).
Kaplan-Meier OS curves of HCC patients according to the status of IL-10, miR-141, and miR-21 levels at the baseline were examined. Figure 2 indicates that the patients in the high IL-10 and the high miR-21 sub-groups showed a poor OS rate compared to those in the corresponding low sub-groups with median OS (95% CI) of 11.90 (6.93–16.87) vs. 19.50 (15.23–23.77) months; P=0.031, and 10.70 (6.47–14.93) vs. 20.70 (16.59–23.81) months; P=0.008, respectively. On the other hand, patients in the high miR-141 sub-group showed a longer OS rate when compared to their corresponding low sub-group with a median OS (95% CI) of 20.33 (15.85–23.82) vs. 11.91 (7.43–16.48) months, P=0.034. These results suggest that high IL-10 level, high miR-21 expression, and low miR-141 expression are associated with poor prognosis of HCC.
Univariate survival analysis was performed with gender, age (dichotomized by median =60 years), tumor size, BCLC stage, IL-10, miR-141, and miR-21 as covariates. The results are reported in Table 3 which shows that only miR-21 was statistically significant (HR =6.09, 95% CI: 1.27–29.15; P=0.024), although IL-10 and miR-141 were borderline significant (HR=3.90; 95% CI: 0.99–15.31; P=0.051 and HR =0.22, CI: 0.05–1.06; P=0.060, respectively). For that, the latter variables only were included in the multivariate analysis that showed the persistence of IL-10 and miR-21 significance (HR =10.22, 95% CI: 1.74–59.91, P=0.010 and HR =12.98, 95% CI: 1.65–102.03; P=0.015, respectively).
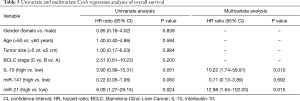
Full table
Discussion
Curcumin was demonstrated by several preclinical studies as a promising anticancer drug (36-38). This anticancer potential may attribute to its myriad biological properties which make it able to suppress the initiation, promotion, proliferation, and metastasis of a wide variety of malignant tumors (39). However, poor bioavailability of curcumin was considered the major obstacle for its clinical application which can be overcome by the co-administration with piperine known to increase curcumin bioavailability in animals and humans (18). In addition, it has been shown that taurine reduces the levels of pro-inflammatory cytokines and exerts an anti-cancer effect by inducing the apoptosis of cancer cells (40,41). Therefore, this study was designed to assess the effect of a combined therapy composed of curcumin, piperine, and taurine, on serum IL-10 level and the expression level of circulating miR-141 and miR-21 in patients with HCC.
The results of the current study revealed that high IL-10 level was a poor prognostic sign where the median OS of patients in the low IL-10 sub-group was 1.6 folds more than that of patients in the high IL-10 sub-group. This echoes previous studies indicating that the serum IL-10 level was an adverse prognostic factor for patients with inoperable HCC and in those after surgical resection (42-44), confirms a predominantly immunosuppressive role of IL-10 for circulating dendritic cells in patients with HCC, and thus may indicate the aspects of tumor immune evasion (45). The combined treatment was able to decrease IL-10 serum level significantly after 2 cycles of administration which can be attributed to the effect of the treatment on the signal transducer and activator of transcription3 (STAT3) which is a pro-inflammatory transcription factor known to be constitutively expressed and play a major role in the pathogenesis of several cancer types (46). In cancer cells, STAT3 promotes the expression of factors that are both immunosuppressive and STAT3 activating, including vascular endothelial growth factor (VEGF) and IL-10. These tumor-derived factors, in turn, up-regulate STAT3 signaling in various immune-cell subsets in the tumor microenvironment, which produce more immunosuppressive factors, including IL-6, IL-10, transforming growth factor-β (TGF-β), and VEGF, thereby abrogating the function of various immune effector cells (47-49). Several studies demonstrated that both curcumin and taurine were able to inhibit STAT3 activation (50-55). Thus, we can speculate that the decreased level of IL-10 upon administration of the combined treatment may be attributed to the negative effect of the treatment on STAT3 activation and consequently on the synthesis and secretion of IL-10.
In the same context, this work showed that the over-expression of the circulating miR-21 was associated with poor prognosis. The median OS of patients in the low miR-21 sub-group was almost the double of that in the high miR-21 sub-group. This is in agreement with the results of prior publications which reported that the OS rate of patients with high miR-21 expression was significantly worse than those of patients with low miR-21 expression in several types of cancers including HCC (56-59). MiR-21 over-expression might enhance HCC migration and invasion through certain mechanisms, resulting in poor survival; it simultaneously regulates multiple programs that promote cell proliferation, apoptosis or tumor invasiveness by targeting phosphatase and tensin homolog (PTEN), programmed cell death 4 (PDCD4), and reversion-inducing cysteine-rich protein with Kazal motifs (RECK) in HCC (60-63). Herein, the expression of miR-21 was down-regulated by the combined treatment which is consistent with the results of Wang et al. (64) who demonstrated that the anticancer effect of curcumin significantly reduced the expression of miR-21 in MCF-7 breast cancer cells in a concentration-dependent manner. Similarly, Mudduluru et al. (65) revealed that curcumin inhibits the invasion and metastasis of colorectal cancer cells through the regulation of miR-21 expression. Furthermore, Tu et al. (66) reported that reactive oxygen species (ROS) promoted gastric carcinogenesis via up-regulating miR-21 expression which in turn down-regulates the expression of PDCD4 in gastric cancer cells. Thus, taurine and because of its antioxidant activity may down-regulate the expression of miR-21 possibly via scavenging free radicals.
In HCC, miR-141 could function as a tumor suppressor by targeting the metastasis-related T lymphoma invasion and metastasis 1 (Tiam1) gene (29). Moreover, a study by Xu et al. (67) suggested that over-expression of miR-141 suppressed HCC cell growth and invasion via targeting the transcription inhibitor zinc finger E-box-binding homeobox 2 (ZEB2). This study revealed that the down-regulation of the circulating miR-141 expression level may contribute to poor prognosis. The median OS of patients in the high miR-141 sub-group was as high as 1.7 folds of that in the low miR-141sub-group. This is in agreement with the results of Lu et al. (68) who showed that the down-regulation of miR-141 may contribute to the aggressive progression and poor prognosis of human gastric cancer. Further, it was found that miR-141 may act as an independent prognostic factor for pancreatic cancer patients (69). Additionally, Wszolek et al. (70) suggested that miR-141 up-regulation could act as an independent indicator for favorable prognosis of bladder cancer. Unfortunately, the combined therapy resulted in a non-significant up-regulation of the circulating miR-141 expression level.
In conclusion, the results of the present work support the notion that altered expression of serum IL-10, miR-21, and miR-141 may be prognostic biomarkers that may help to guide treatment in HCC. Further, in a population of patients with fairly advanced HCC, the administration of a combined treatment composed of curcumin, piperine, and taurine for three successive months was able to decrease the circulating level of IL-10 and miR-21, with no effect on miR-141 expression level, which may be reflected on the OS rate positively.
Study limitation
There were several limitations to our study. First, this was a single-center study so there are likely selection bias and limitation of the generalizability of the findings. Second, this was a preliminary study performed on a small sample size and more subjects would provide stronger statistical analyses. Finally, the evaluation in a randomized trial may be warranted to further delineate the potential clinical benefits and risks.
Despite these limitations, this study still had its own special advantages; to the best of our known, this is the first study that analyzed the effect of such a treatment on HCC patients which may open the way to new and promising therapeutic strategies. Additionally, if confirmed in further studies, this study showed that serum IL-10 and miR-21 may represent non-invasive biomarkers of the therapeutic response at least in terms of OS rate.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This work was carried out in accordance with The Declaration of Helsinki for experiments involving humans and the study protocol was approved by the institutional review board of NCI (Organization No. IORG0003381) under the number of IRB00004025 with a Federalwide Assurance (FWA) number of FWA00007284. Informed consent was obtained from all participants before participating in the study.
References
- Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis 2009;27:80-92. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Azzam AZ. Liver transplantation as a management of hepatocellular carcinoma. World J Hepatol 2015;7:1347-54. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Livraghi T. Percutaneous ethanol injection in hepatocellular carcinoma. Digestion 1998;59:80-2. [Crossref] [PubMed]
- Giorgio A. Percutaneous radiofrequency ablation of hepatocellular carcinoma on cirrhosis: state of the art and future perspectives. Recent Pat Anticancer Drug Discov 2010;5:69-76. [Crossref] [PubMed]
- Boutros C, Somasundar P, Garrean S, et al. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol 2010;19:e22-32. [Crossref] [PubMed]
- Li Y, Kong D, Wang Z, et al. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res 2010;27:1027-41. [Crossref] [PubMed]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as ‘Curecumin”: from kitchen to clinic. Biochem Pharmacol 2008;75:787-809. [Crossref] [PubMed]
- Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol 2011;2:1-14. [Crossref] [PubMed]
- Abouzied MM, Eltahir HM, Abdel Aziz MA, et al. Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT, and caspase-3 expression in experimental rat model. Tumour Biol 2015;36:1763-71. [Crossref] [PubMed]
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895-900. [PubMed]
- Shehzad A, Khan S, Shehzad O, et al. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today (Barc) 2010;46:523-32. [Crossref] [PubMed]
- Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 2014;46:2-18. [Crossref] [PubMed]
- Mittal R, Gupta RL. In vitro antioxidants activity of piperine. Methods Find Exp Clin Pharmacol 2000;22:271-4. [Crossref] [PubMed]
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 2007;47:735-48. [Crossref] [PubMed]
- Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed 2013;3:253-66. [Crossref] [PubMed]
- Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353-6. [Crossref] [PubMed]
- Suresh D, Srinivasan K. Tissue distribution and elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res 2010;131:682-91. [PubMed]
- Sehgal A, Kumar M, Jain M, et al. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum Exp Toxicol 2012;31:473-82. [Crossref] [PubMed]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev 1992;72:101-63. [Crossref] [PubMed]
- Wang L, Zhao N, Zhang F, et al. Effect of taurine on leucocyte function. Eur J Pharmacol 2009;616:275-80. [Crossref] [PubMed]
- Yu JS, Kim AK. Effect of combination of taurine and azelaic acid on antimelanogenesis in murine melanoma cells. J Biomed Sci 2010;17:S45. [Crossref] [PubMed]
- Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 2008;14:109-19. [Crossref] [PubMed]
- Othman MS, Aref AM, Mohamed AA, et al. Serum levels of interleukin-6 and interleukin-10 as biomarkers for hepatocellular carcinoma in Egyptian patients. ISRN Hepatol 2013;2013:412317. [Crossref] [PubMed]
- Kim DY, Kim JW, Kuromatsu R, et al. Controversies in surveillance and early diagnosis of hepatocellular carcinoma. Oncology 2011;81:56-60. [Crossref] [PubMed]
- Zekri AR, Ashour MS, Hassan A, et al. Cytokine profile in Egyptian hepatitis C virus genotype-4 in relation to liver disease progression. World J Gastroenterol 2005;11:6624-30. [Crossref] [PubMed]
- Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol 2014;9:287-314. [Crossref] [PubMed]
- Liu Y, Ding Y, Huang J, et al. miR-141 suppresses the migration and invasion of HCC cells by targeting Tiam 1. PloS One 2014;9:e88393. [Crossref] [PubMed]
- Lin L, Liang H, Wang Y, et al. MicroRNA-141 inhibits cell proliferation and invasion and promotes apoptosis by targeting hepatocyte nuclear factor-3β in hepatocellular carcinoma cells. BMC Cancer 2014;14:879. [Crossref] [PubMed]
- Wu SM, Ai HW, Zhang DY, et al. MiR-141 targets ZEB2 to suppress HCC progression. Tumour Biol 2014;35:9993-7. [Crossref] [PubMed]
- Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007;133:647-58. [Crossref] [PubMed]
- Zhang H, Ozaki I, Mizuta T, et al. Involvement of programmed cell death 4 in transforming growth factor- beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 2006;25:6101-12. [Crossref] [PubMed]
- Zhu S, Si ML, Wu H, et al. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 2007;282:14328-36. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 2007;67:3853-61. [Crossref] [PubMed]
- LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 2005;11:6994-7002. [Crossref] [PubMed]
- Uddin S, Hussain AR, Manogaran PS, et al. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene 2005;24:7022-30. [Crossref] [PubMed]
- Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin: a short review. Life Sci 2006;78:2081-7. [Crossref] [PubMed]
- Akay C, Yaman H, Oztosun M, et al. The protective effects of taurine on experimental acute pancreatitis in a rat model. Hum Exp Toxicol 2013;32:522-9. [Crossref] [PubMed]
- Tu S, Zhang X, Luo D, et al. Effect of taurine on the proliferation and apoptosis of human hepatocellular carcinoma HepG2 cells. Exp Ther Med 2015;10:193-200. [Crossref] [PubMed]
- Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg 2000;231:552-8. [Crossref] [PubMed]
- Hattori E, Okumoto K, Adachi T, et al. Possible contribution of circulating interleukin-10 (IL-10) to antitumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res 2003;27:309-14. [Crossref] [PubMed]
- Chan SL, Mo FK, Wong CS, et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 2012;118:3984-92. [Crossref] [PubMed]
- Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res 2004;10:7260-9. [Crossref] [PubMed]
- Aggarwal BB, Sethi G, Ahn KS, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci 2006;1091:151-69. [Crossref] [PubMed]
- Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004;10:48-54. [Crossref] [PubMed]
- Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 2003;22:319-29. [Crossref] [PubMed]
- Kinjyo I, Inoue H, Hamano S, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β1. J Exp Med 2006;203:1021-31. [Crossref] [PubMed]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol 2003;171:3863-71. [Crossref] [PubMed]
- Pandey A, Vishnoi K, Mahata S, et al. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-Fluorouracil. Nutr Cancer 2015;67:1293-304. [Crossref] [PubMed]
- Fetoni AR, Paciello F, Mezzogori D, et al. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer 2015;113:1434-44. [Crossref] [PubMed]
- Weissenberger J, Priester M, Bernreuther C, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res 2010;16:5781-95. [Crossref] [PubMed]
- Kim KS, Ji HI, Chung H, et al. Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids 2013;45:1415-22. [Crossref] [PubMed]
- Nakajima Y, Osuka K, Seki Y, et al. Taurine reduces inflammatory responses after spinal cord injury. J Neurotrauma 2010;27:403-10. [Crossref] [PubMed]
- Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008;14:2348-60. [Crossref] [PubMed]
- Hu A, Huang JJ, Xu WH, et al. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: association with patient survival. Am J Transl Res 2014;6:604-13. [PubMed]
- Wang WY, Zhang HF, Wang L, et al. MiR-21 expression predicts prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2014;38:715-9. [Crossref] [PubMed]
- Karakatsanis A, Papaconstantinou I, Gazouli M, et al. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog 2013;52:297-303. [Crossref] [PubMed]
- Zhou L, Yang ZX, Song WJ, et al. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol 2013;43:661-9. [Crossref] [PubMed]
- Liu C, Yu J, Yu S, et al. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol 2010;53:98-107. [Crossref] [PubMed]
- Bao L, Yan Y, Xu C, et al. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett 2013;337:226-36. [Crossref] [PubMed]
- Zhu Q, Wang Z, Hu Y, et al. MiR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol Rep 2012;27:1660-8. [PubMed]
- Wang X, Hang Y, Liu J, et al. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol Lett 2017;13:4825-31. [Crossref] [PubMed]
- Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep 2011;31:185-97. [Crossref] [PubMed]
- Tu H, Sun H, Lin Y, et al. Oxidative stress upregulates PDCD4 expression in patients with gastric cancer via miR-21. Curr Pharm Des 2014;20:1917-23. [Crossref] [PubMed]
- Xu H, Mei Q, Xiong C, et al. Tumor-suppressing effects of miR-141 in human osteosarcoma. Cell Biochem Biophys 2014;69:319-25. [Crossref] [PubMed]
- Lu YB, Hu JJ, Sun WJ, et al. Prognostic value of miR-141 downregulation in gastric cancer. Genet Mol Res 2015;14:17305-11. [Crossref] [PubMed]
- Zhao G, Wang B, Liu Y, et al. MiRNA-141, downregulated in pancreatic cancer, inhibits cell proliferation and invasion by directly targeting MAP4K4. Mol Cancer Ther 2013;12:2569-80. [Crossref] [PubMed]
- Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol 2011;29:794-801.e1. [Crossref] [PubMed]


