
Timing of surgery following neoadjuvant chemoradiation in rectal cancer: a retrospective analysis from an academic medical center
Introduction
Colorectal cancer is the third most common cancer worldwide with approximately 43,000 new diagnoses each year in the United States (1). Cause of death is routinely related to distant metastatic disease, necessitating efforts to curb risk for progression of early stage disease (2). Standard of care for early stage disease is a multidisciplinary approach involving use of neoadjuvant chemoradiation followed by surgical resection. More recently, timing for rectal cancer surgery after neoadjuvant chemoradiotherapy (nCRT) has been studied as an independent variable that may influence perioperative complications, risk for local recurrence (LR), and overall survival (OS).
The landmark Lyon trial was the first reported randomized controlled trial to study the time interval from radiation therapy to surgery as a variable for improved long-term outcomes (3). The trial demonstrated that by waiting a median of 46 days from the end of radiation therapy (without chemotherapy), the likelihood of clinical and pathologic downstaging increases without sacrificing postoperative complications. However, the study did not show a significant difference in survival or local control between longer and shorter interval times to surgery. Following this trial, surgery 4–8 weeks after neoadjuvant therapy was confirmed to be safe and soon became the standard of care. Numerous subsequent retrospective studies have investigated the question of the ideal interval between neoadjuvant therapy and surgery with mixed results (4-10). Most recently, a randomized trial comparing 7- vs. 11-week delays in surgery showed no difference in pathologic complete response (pCR), but higher morbidity and more difficult surgical resection in those who were delayed beyond 11 weeks (11).
Despite these studies, the optimal timing of surgery after nCRT, in terms of OS, disease-free survival (DFS), and perioperative morbidity remains unknown. We analyzed the impact of timing of surgery on perioperative morbidity and OS at our institution over a 12-year period
Methods
Patients
The Iowa Cancer Registry (ICR) is a population-based cancer registry for the state of Iowa and is a member of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. This registry was queried to identify all patients with rectal cancer between 2000 and 2012. The use of ICR data and electronic medical records was approved by the University of Iowa Institutional Review Board (IRB) and by an independent ethic committee per local regulations. Rectal cancer patients were included for retrospective review if they had biopsy-confirmed rectal adenocarcinoma involving the proximal and distal rectum with locally advanced disease and absence of metastatic disease. Patients who did not undergo nCRT were also excluded. As the University of Iowa Hospitals and Clinics is a large referral center for the state of Iowa, some patients received diagnostic evaluations and neoadjuvant therapies at outside hospitals, but all analyzed patients underwent surgery at our hospital. Electronic medical records of the final cohort were then reviewed.
Clinical and surgical data
Tumor staging was determined by physical exam, endoscopic evaluation and imaging techniques including computed tomography (CT) scan, positron emission tomography (PET) scan, and magnetic resonance imaging (MRI). Type of chemotherapy, radiation therapy technique, and time to surgery were not standardized; thus, all methods were included in the study. Chemotherapy comprised of infusional or oral chemotherapy regimens with dosing guided by National Comprehensive Cancer Network (NCCN) guidelines. Infusional chemotherapy in the form of 5-flurouracil (5-FU) consisted of one of two regimens: (I) 5-FU 400 mg/m2 plus leucovorin 200 mg/m2 bolus on days 1–4 of weeks 1 and 5, or (II) 225 mg/m2/day 5-FU IV continuous infusion 5–7 days per week during radiation. Patients who received the oral equivalent were given capecitabine at a twice-daily dose of 825 mg/m2 on days of radiation throughout the entire duration of radiotherapy. Radiation therapy techniques included use of 3-dimensional conformal radiotherapy (3D-CRT) and intensity modulated radiation therapy (IMRT) with 50.4 Gy over 25 fractions delivered over 5 weeks. Time of surgery was determined by the surgeon at the post-nCRT follow-up where response to CRT was determined by repeat staging work-up. Patients with metastatic disease at this time was excluded. All surgeries were performed by experienced colorectal surgeons who exclusively performed total mesorectal excisions. Techniques included abdominal peritoneal resection (APR), low anterior resection (LAR) with colorectal or coloanal anastomosis, or total pelvic exenteration. Surgical specimens were examined grossly and microscopically by trained pathologists who assessed tumor extent according to TNM criteria. All lymph nodes retrieved were examined histologically and all nodal deposits larger than 3 mm in diameter were considered positive. All postoperative pathology slides were re-reviewed by a single pathologist for this study.
Endpoints
Primary endpoints were OS, defined as number of weeks from start of nCRT to date of death or last follow-up, and time interval (TI) to surgery, defined as weeks from last day of CRT to day of surgery. All patients were divided into two groups based on TI: those undergoing surgery within 8 weeks of completing nCRT and those beyond 8 weeks. Secondary endpoints included pCR, tumor downstaging, hospital length of stay (LOS) following surgical procedure, intraoperative blood loss, and major postoperative complications including wound infection, surgical site infection, anastomotic leak, and small bowel obstruction or ileus within the first 30 days of surgery. Surgical site complications were defined as any anastomotic or wound complication, or both. The rate of anastomotic leakage was determined by clinically directed imaging investigations, and if needed, on intraoperative findings during repeat laparotomy/laparoscopy. Wound infection was defined by documentation per the primary surgical team. Patient characteristics and treatment regimens were also studies.
Statistical analysis
TI was calculated for each patient using electronic medical records. Univariate Cox proportional hazard models were used to study the association between TI and OS to help define the optimal interval between chemoradiotherapy and surgery. Survival probabilities were estimated and plotted using the Kaplan-Meier method. Associations of TI with the secondary endpoints defined above were tested using Chi-square tests for categorical covariates and ANOVA for numerical covariates. All testing was performed on the univariate level and unadjusted for multiple comparisons due to the retrospective nature of the study. Differences between survival curves were compared using the log-rank test. All statistical testing was two-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 87 patients presented with stage II or III rectal cancer. Patient, neoadjuvant treatment and surgical characteristics are presented in Table 1. Median age was 55 years old (range, 25–84 years old). The majority did not have diabetes (87.4%), hypertension (69.0%), or dyslipidemia/coronary artery disease (85.1%). Approximately 80.5% of patients received infusional 5-FU while 19.5% received capecitabine. Neoadjuvant chemoradiation was well-tolerated as few patients suffered from rectal pain (14.9%), serositis/mucositis (14.9%), or nausea/vomiting (11.5%). In total, 68 of 87 patients were able to complete chemotherapy as planned, but 10 suffered from toxicities resulting in absolute discontinuation.
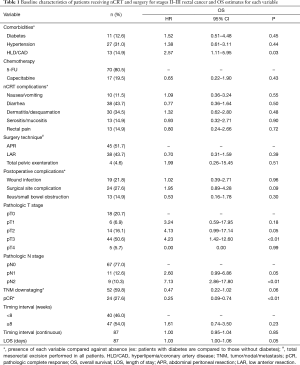
Full table
Mean TI was 9.92 weeks (range, 3 days to 94 weeks). A single patient with almost 2 years between neoadjuvant therapy and surgery elected to postpone surgery after having achieved complete clinical response; surgery was eventually performed after minimal disease was found on routine colonoscopy surveillance.
Surgery was performed at TI <8 weeks in 46.0% of patients. APR was performed in 45 (51.7%), while LAR was used in 38 (43.7%), and total pelvic exenteration was reserved for 4 patients (4.6%; Table 1). In total, 63 of 87 patients (72.4%) suffered no perioperative surgical site complications while 24 patients (27.6%) encountered at least one documented complication. The incidence of post-anastomotic leak was 18.4% (7 of 38) while the incidence of postoperative wound infection/dehiscence was 21.8% (19 of 87; Table 1). In total, 52 of 87 patients (59.8%) exhibited evidence of tumor downstaging following neoadjuvant therapy, and 24 of 87 (27.6%) achieved pCR. Furthermore, the rate of pCR was 27.5% in those undergoing surgery at TI <8 weeks vs. 27.7% in those with TI ≥8 weeks.
Univariate Cox proportional hazard models revealed no significant association between OS and TI when comparing TI <8 weeks to ≥8 weeks (P=0.23; Table 1) or when considering the interval as a continuous variable (P=0.85; Table 1). Increased LOS [median 7.00 days, P=0.05, HR 1.03 (1.00–1.06)] correlated with worse survival outcomes, as did postoperative T3 tumor stage (P<0.01) and postoperative N2 node status (P<0.01). T4 tumor stage did not yield any clinically significant correlation (P=0.99) but also had a small sample size (n=5; Table 1). pCR was associated with improved OS (P<0.01) while tumor downstaging was not significant (P=0.06). Despite the significance with OS, pCR was not influenced by TI (P=0.99), nor was incidence of tumor downstaging (P=0.40; Table 2). Delaying surgery beyond 8 weeks was associated with a statistically significant increased risk for wound infection (P=0.05). There was no significant association between TI and other surgical complications, including surgical site complication (P=0.33), postoperative ileus/small bowel obstruction (P=0.54), or blood loss (P=0.44; Table 2); however, patients undergoing pelvic exenteration experienced notably longer LOS (n=4, mean 24.25 days, max 60 days).
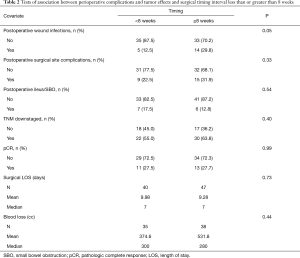
Full table
Discussion
Use of nCRT is currently the standard of care in locally advanced rectal cancer (12), but there is no consensus as to the optimal timing of surgery after nCRT. Historically, delaying surgery 4–8 weeks has been widely accepted (3). Despite no randomized trial confirming benefit, intervals beyond 8 weeks have been utilized. In our study, the average timing interval was 10 weeks which is consistent with this trend.
Numerous retrospective studies and a single prospective study have shown significant improvement in pCR with TI ≥8 weeks (13-18). In their prospective study, Garcia-Aguilar et al. performed a non-randomized, prospective study comparing 6- to 11-week TI, with those in the longer interval also receiving two additional cycles of FOLFOX. Whether the improved pCR (25% vs. 18%) in the 11-week cohort is due to the delayed interval or the additional chemotherapy cannot be conclusively determined by this study (18). Probst et al. performed a meta-analysis illustrating improved pCR in those undergoing surgery ≥8 weeks after nCRT (5), while another group showed the most significant pCR and T/N downstaging in the 10- to 11-week group (6). In contrast, other studies have shown less favorable support of delayed surgery, by finding no difference in pCR, OS, DFS, or LR (4,7,8-10). Stein et al. retrospectively studied 40 patients, comparing 4- to 8-week and 10- to 14-week intervals. They identified a trend toward benefit in pCR, LR and sphincter preservation with shorter intervals; however, because of the small sample size, statistical significance was not met (19).
Our results demonstrated that TI before or after 8 weeks did not influence OS, which is compatible with findings from prior investigators. The Lyon trial is the only reported RCT to date to examine impact of timing interval on OS. It did not show improved OS or LR with longer intervals (3). Tulchinsky et al. retrospectively demonstrated no significant difference in OS in patients with surgical interval <7 vs. >7 weeks (15). In comparison, other groups have been able to show survival benefit with a longer interval (>7–8 weeks) (14,16).
Factors that influenced OS in our cohort included pathologically staged T3 and N2 disease, with larger tumors and greater nodal burden linked with poorer outcomes. Evidence of larger residual tumor burden and nodal involvement following nCRT likely reflects the intrinsic tumor biology with chemotherapy-resistant tumors portraying worse outcomes. In addition, larger tumors and residual nodal burden may also function as a surrogate to tumor downstaging and pCR. Tumor downstaging from neoadjuvant treatment was not statistically significant (P=0.06) but trended toward significance. pCR was associated with improved OS (P<0.01). Our results indicate a pCR of 27.6% for our entire cohort. In a study with more than 17,000 rectal cancer patients, Probst et al. retrospectively showed a pCR of 8.7% before 6 weeks TI and 13.2% after 8 weeks (5). In recent RCTs, pCR has varied between 9% and 17% (11,20-23). Our pCR is higher than comparable studies, but some of this discrepancy may be a function of the number of patients in the cohorts. Despite the improved rate, our findings did not confirm that pCR was influenced by TI. While some studies have drawn similar conclusions (4,24), others have demonstrated higher pCR rate being associated with longer surgical intervals (14,15,25). Thus, the optimal interval that maximizes rate, and the significance of pCR itself, has yet to be determined.
In our study, longer intervals between nCRT and surgery was associated with an increased risk of postoperative wound infections, but did not carry increased risk for anastomotic failure, postoperative ileus, blood loss, or LOS. Our data contrasts with the Lyon trial which found no difference in perioperative complications between the shorter and longer intervals, but their definition of the latter did not extend beyond 8 weeks. In the only randomized trial studying postoperative complications at TI beyond 8 weeks, longer intervals were associated with increased morbidity (11). Patients who underwent surgery after 11 weeks had a technically more difficult resection, and higher rates of perineal healing problems were observed after APR.
The relationship between TI and surgical morbidity has also been studied retrospectively. One group favored surgeries beyond 8 weeks because of increased risk for anastomotic leakage and perineal wound infection with intervals less than 8 weeks (7). However, this analysis was influenced by a high rate of postoperative complications including anastomotic failure (26.8%) and perineal wound complications (34.2%). In comparison, several groups have reported no difference in postoperative morbidity (13,19,26,27), while others have shown increased risk for perioperative complications with delayed intervals, similar to our findings (9). This risk has been rationalized by increased post-radiation intrapelvic fibrotic changes leading to more difficult dissection and/or more friable mesorectal tissue. The risk for wound infection could be explained by similar factors as a longer interval leads to more cellular lysis of healthy tissue which poses risk for wound dehiscence and intraabdominal or perineal infections.
Our postoperative rates for anastomotic failure, surgical site complications, and wound infections were 22.5%, 27.6%, and 21.8%, respectively, which is similar to prior studies (7,15). A prolonged LOS after surgery was not influenced by TI but was associated with diminished OS. In our cohort, four patients had surgical complications necessitating pelvic exenteration. Given such a small number undergoing exenteration, no significant associations can be assessed, but patients undergoing this surgery experienced notably longer LOS (n=4, mean 24.25 days, max 60 days). Although the incidence of major postoperative complications were rare, such occurrences notably cost patients and the healthcare system significant resources; thus, our results implicate potential economic benefits to targeting surgery before 8 weeks.
There are several limitations to our study. First, as a retrospective study, we are unable to control for TI. Ultimately, the decision for surgery depends on the individual surgical oncologist, and patients received their care from a variety of surgeons at our institution. Secondly, our population of 87 patients is smaller than those in other retrospective studies. The number of patients was partially limited by incomplete available data in the Iowa Cancer Registry and the electronic medical record, stemming largely from patients obtaining neoadjuvant therapy at local hospitals from which we were unable to obtain IRB approval for retrieval. Lastly, as we only studied timing to surgery in relation to neoadjuvant therapies, we are unable to discern how postoperative therapies could have influenced outcomes. However, given currently limited prospective data investigating the relationship between surgical TI and OS, we believe our analysis to be valuable.
Conclusions
OS was not significantly impacted by longer intervals between neoadjuvant chemoradiation and surgery. Delaying surgery beyond 8 weeks was associated with increased risk for wound infection.
Acknowledgments
We would like to thank Kris Greiner for her assistance with preparation of the final manuscript. We would also like to thank Dr. Lai Xu and Dr. Agnes Ounda for assistance with data extraction for the medical records.
Funding: This project received funding from NCI P30 CA86862.
Footnote
Conflicts of Interest: This work was presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, 2018 (J Clin Oncol 2018;36:702).
Ethical Statement: The study was approved by the University of Iowa Institutional Review Board (IRB number: 201301819) and by an independent ethic committee per local regulations. As a retrospective chart review, written informed consent from subjects was waived.
References
- American Cancer Society. Key Statistics for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal Cancer. Lancet 2010;375:1030-47. [Crossref] [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396. [Crossref] [PubMed]
- Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008;248:243-51. [Crossref] [PubMed]
- Probst CP, Becerra AZ, Aquina CT, et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg 2015;221:430-40. [Crossref] [PubMed]
- Sloothaak DA, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933-9. [Crossref] [PubMed]
- Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg 2008;95:1534-40. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys 2008;71:1181-8. [Crossref] [PubMed]
- Huntington CR, Boselli D, Symanowski J, et al. Optimal timing of surgical resection after radiation in locally advanced rectal adenocarcinoma: an analysis of the National Cancer Database. Ann Surg Oncol 2016;23:877-87. [Crossref] [PubMed]
- Supiot S, Bennouna J, Rio E, et al. Negative influence of delayed surgery on survival after preoperative radiotherapy in rectal cancer. Colorectal Dis 2006;8:430-5. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Rectal Bone Cancer (Version 3.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 2004;47:279-86. [Crossref] [PubMed]
- Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 2009;250:582-9. [PubMed]
- Tulchinsky H, Shmueli E, Figer A, et al. An interval 7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7. [Crossref] [PubMed]
- Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833-41. [Crossref] [PubMed]
- Zeng WG, Zhou ZX, Liang JW, et al. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol 2014;110:463-7. [Crossref] [PubMed]
- Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 2011;254:97-102. [Crossref] [PubMed]
- Stein DE, Mahmoud NN, Anné PR. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum 2003;46:448-53. [Crossref] [PubMed]
- Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [Crossref] [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638-44. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [Crossref] [PubMed]
- Jeong DH, Lee HB, Hur H, et al. Optimal timing of surgery after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Korean Surg Soc 2013;84:338-45. [Crossref] [PubMed]
- Petrelli F, Coinu A, Lonati V, Barni S. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis 2015;30:447-57. [Crossref] [PubMed]
- Tran CL, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006;192:873-7. [Crossref] [PubMed]
- Bujko K, Nowacki MP, Kepka L, et al. Postoperative complications in patients irradiated pre-operatively for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs chemoradiation. Colorectal Dis 2005;7:410-6. [Crossref] [PubMed]


