
MicroRNA-122 in patients with hepatitis B and hepatitis B virus-associated hepatocellular carcinoma
Introduction
Hepatitis B virus (HBV) is known as a small enveloped virus with a partially double-stranded 3.2 kb DNA genome belonging to the Hepadnaviridae family (1). HBV infection is also considered as one of the main causes of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) (2). Approximately 350 million people across the world are estimated to be persistent carriers of HBV and most of them may have a chronic hepatic disease (3). Recently, microRNA (abbreviated as miRNA) family has been taken into consideration in virology research. It should be noted that miRNAs are conserved in their evolutions; in this regard, small RNAs (18–25 ribonucleotide) are non-coding and they are able to regulate numerous biological processes such as cellular evolution, differentiation, proliferation, apoptosis, as well as metabolism (4). Moreover, they can play a significant role in pathogenesis of chronic inflammation and cancer (5,6). Various cellular changes can be also mediated by miRNA expression indicating that miRNA expression is able to improve pathogenesis understanding of HBV and make new methods for HBV treatment. Besides, miRNA molecules are used as a prognostic and diagnostic factor for disease assessment. Recent studies in this domain have suggested that miRNAs are manifold in the liver and they can regulate a wide range of its functions. Some of the miRNAs found in serums and tissues can similarly have a role in diagnosis of HBV infection-related tumors (7,8). MiRNA dysregulation is likely to occur in all the stages of hepatocarcinogenesis. Moreover, miRNA profiles can differentiate between healthy people and those affected with HCC (9,10). Such profiles are also different in benign and malignant tissues and they may be different in terms of malignancy by their sub-type (11). MiRNAs can be diagnosed in hepatic tumors, serums, plasmas, and urines which can be considered as a non-invasive method for evaluating responses towards treatments and a kind of prognosis in this disease. As well, etiology-related differences in miRNA expression may complicate efforts to develop effective biomarker panels due to geographic differences in the underlying causes of HCC. Pathogeneses of diseases related to HBV are unknown and treatment strategies in this regard are not sufficient. Multiple studies have indicated that HBV infection can change cellular expressions of miRNAs. Moreover, various miRNAs are able to express themselves in different stages of HBV; for example, some miRNAs can have a significant role in early hepatic tumors and metastasis. Recently, some reports have revealed that miRNAs can be utilized as a marker for hepatic diseases and HBV infections (12-15). The selected and valid miRNA markers are also applied on a wide range of serums and plasmas of HBV-infected patients along with healthy individuals. A combination of these experimental results has been also applied on rat models with a hepatic disease. The results of this application have suggested that plasma concentration changes of miRNA-122 could be dependent on severity of the disease in rat and human models. The changes in miRNA-122 can occur earlier than those of aminotransferase activities. The related studies have similarly indicated that miRNA-122 can have a potential to be used as a blood marker in hepatic diseases such as HBV (14). In this regard, Waidmann et al. analyzed the relationship between miRNA-122 and HBV infection. They showed that a serum level of miRNA-122 was able to differentiate between healthy people and HBV-infected ones (12,13). Some of the circulating miRNA changes also happened due to the severity of HBV symptoms and miRNA-122 expression in these patients could be reported significantly higher than that in healthy individuals (15). Since miRNA-122 has a high expression in the liver and tumor suppressor-like qualities, researchers are interested to know whether miRNA-122 expression can change under HBV hepatic disease in serums on not. MiRNA-122 is located in the intragenic region of 18q21.31 (16). According to the significant role of miRNA-122 in liver physiology, its expression can be suppressed by HBV, which may prevent hepatic disorders and control hepatic diseases such as HCC. Since miRNA-122 has a central role in liver disease and biology, it can be a significant treatment target for hepatic diseases such as hepatitis, fibrosis, steatosis (fatty change), and HCC. Furthermore, studies conducted in this domain have reported that miRNA-122 suppression can be an efficient method for decreasing viral loads in HBV patients. The research on miRNA-122 has also continued as a treatment and a diagnosis target for HBV infection.
MiRNA-122 is the most abundant among exclusive hepatic miRNAs and it makes 70% of all the colonized miRNAs from the liver (17). In this respect, studies have indicated that miRNA-122 can have a key role in controlling the growth of hepatocytes and neoplastic transformations (18). It can also play a significant role in regulating metabolisms, lipids, hepatic cirrhosis, and HBV replication changes (19-21). Such studies have similarly showed that miRNA-122 can have a suppression effect on HBV replication. Moreover, interaction between miRNA-122 expression levels and replication of HBV has demonstrated the suppression effects of miRNA-122 on HBV via base-pairing of HBV sequences (22,23). In this respect, miRNA-122 can control HBV through down-regulating Cyclin G1 and increasing p53 activities (23). The serum miRNA-122 levels can be also correlated with the serum levels of ALT, HBV DNA, and HBsAg in clinical settings (13,24). Therefore, patients with high levels of HBsAg can be exposed to viral flare-up; moreover, their serum miRNA-122 level can be reported high. According to the fact that miRNA-122 is able to increase the quickness of hepatitis C virus (HCV) replication and suppress HBV one, the potentials of miRNA-122 in patients infected with HBV and HCV is an interesting issue for clinical analyses. In cultured cells, HBV infection is likely to decrease or increase the expression of miRNA-122 leading to lower HBV replication (22). Furthermore, an inverse linear relationship can be observed between miRNA-122 levels and viral loads in peripheral-blood mononuclear cells of HBV patients (22). In this line, research studies have reported that HBV infection can increase the level of pri-miRNA-122, but decrease the level of miRNA-122 since HBV sequences can have several binding sites for miRNA-122 (25). The reason for the high serum levels of miRNA-122 in patients with high HBsAg has not become clear yet. However, one of the potential reasons is that intracellular miRNA-122 is carried out into the serum by virus or host proteins. Therefore, miRNA-122 level can increase in the serum. Argonaut 2 and HBsAg have been also proved to have the ability of carrying miRNAs—such as miRNA-122—into the blood circulation (15,26). Besides, the increase of miRNA-122 in serum can be created by released miRNA-122 from the damaged hepatocytes caused by inflammation (14). Anti-tumor functions of miRNA-122 in the liver can be also analyzed using miRNA-122 KO mice (27,28). These rat models can spontaneously progress liver tumors and show abnormal expression of genes involved in cell growth, cell death, and epithelial-mesenchymal transition (EMT) (27,28). Tumor progression in these mice can be also reduced as the expression of miRNA-122 increases (27,28). Furthermore, using a mouse model where tumors are developed despite the absence of inflammation, it has been revealed that miRNA-122 can have an anti-tumor function independent of its role in inhibiting liver disease and inflammation (27).
MiRNA-122 and hepatitis B-associated HCC
MiRNA-122 can have a significant role in EMT of hepatic cells and HCC progression (28,29). The level of miRNA-122 is down in HCC, and the low level of miRNA-122 can be a poor prognosis in this type of cancer (30,31). In an in vivo experiment, overexpression of miRNA-122 could reduce tumorigenic properties of HCC cells indicating the fact that miRNA-122 could be assumed as a tumor suppressor (32). Furthermore, hydrodynamic injection of miRNA-122 into 3-month old miRNA-122-deficient mice with problems in miRNA-122 expression could bring about a disorder in tumor progression and, eventually, cause a decrease in size and occurrence of the tumor (28). Intra-tumor injection of miRNA-122 encapsulated in nanoparticles of cationic lipid could also reduce the growth of HCC Xenograft up to 50% and consequently suppress the targeted genes along with angiogenesis. Increasing the characteristics of miRNA-122 expression on the tumor, could reverse the hepatoma and prevent the formation of HCC in vivo conditions (27,28,32-36). According to this issue, the potential future strategies for therapies by restoring the expression of miRNA-122 in order to prevent or treat HCC in patients with low levels of miRNA-122 can be appropriate ones for HCC. Thus, miRNA-122 can be a potentially suitable treatment for HCC. In fact, since the reduction of miRNA-122 can cause hepatocarcinogenesis, increasing the expression of miRNA-122 in HCC cells is able to change tumor characteristics of these cells and prevent the progression of HCC in vivo (27,28). It should be noted that miRNA-122 expression in human HCC is low. The increase of miRNA-122 expression can also prevent HCC growth; furthermore, it can promote cellular apoptosis by affecting the signaling path of WNT/b-Catenin-TCF (37) which has a binding site for miRNA-122 and can function as a sponge for it (25). Moreover, a recent study indicated that HBx protein could be a binding to PPARC and inhibit the transcription of miRNA-122 (38). It was also able to decrease miRNA-122 stability via reducing the amount of Gld2 production, which can be of significance in miRNA-122 adenylation (39). It has been reported that the HBV-mediated downregulation of miRNA-122 can also increase the expression of the tumor promoter N-myc downstream regulated gene 3 (NDRG3) (40) which can further enhance the expression of the miRNA-122 target Cyclin G1 (CCNG1) and result in improved Akt activation leading to EMT (41). MiRNA-122 inhibition by HBV can also increase PTTG1 which can affect the growth of the tumor and cell invasion (25). Besides, these HBV changes in regulatory networks can cause the progression of HCC.
Role of miRNA-122 in molecular pathogenesis of HBV
Some types of proteins targeted by miRNA-122 have been identified. The signaling path of WNT/b-catenin is also active in 30% of HCCs (42,43) which are directly targeted by miRNA-122 (37,44). The decrease of WNT1 by miRNA-122 can also lead to a down-regulation of b-catenin expression and reduction of the WNT/b-catenin signaling (37,44). RhoA, a Ras homolog gene family member, can be a potential target of miRNA-122. MiRNA-122 may directly block the EMT by inhibiting RhoA. It can also target insulin growth factor (IGF)-1R, and consequently, reduce IGF-1R/AKT signaling. This process can reduce the activity of glycogen synthase kinase-3b (GSK-3b) and cellular growth (45,46). MiRNA-122 can also inhibit HBV replication by modulating the expression of type-I interferon (IFN), which can have a significant role in host antiviral responses, such as protection from HBV infection (47). The activity of JAK/STAT signaling path can be negatively regulated by SOCS. MiRNA-122 can also down-regulate SOCS3. An increase of SOCS3 expression in chronic hepatitis B (CHB) patients is likely to produce inefficient immunity, which causes viral persistence (48). Moreover, the hepatic SOCS3 level can be higher in CHB patients, especially in those individuals who do not respond to IFN treatments (49-52). The decrease of miRNA-122 expression in HBV can similarly increase the expression of cyclin G1 gene. Then, cyclin G1 can attenuate the activity of P53, which increases HBV replication. Therefore, loss of miRNA-122 expression in HBV patients may activate modulating cyclin G1 and increase HBV replication (23,25). HBx-LINE1 is a combination of human LINE1 and HBx gene of HBV. In HCC tumor cells, HBx-LINE1 can act as a sponge for miRNA-122 and remove it from the cell. It also activates B-Catenin signaling of hepatic cells, reduces E-cadherin, increases cell migration, and produces an abnormal mitosis and hepatic damage in rats (53).
Polymorphisms miRNA-122 in HBV
The 5'UTR of the target mRNAs can have a significant role in inflammatory diseases caused by HBV and hepatocarcinogenesis (54,55). Polymorphism in the binding site of miRNA to mRNA can also change the binding power of miRNA and regulate target genes; therefore, it can affect patients’ sensitivity to cancer (56). In a study, it was reported that polymorphism in the binding site of miRNA-122 in IL-1A gene could have a risk of HCC (57). The variant allele of rs3783553 could also significantly decrease the cancer-promoting effect of HBV preS deletion in the HBV-infected subjects. These results could contribute to understanding the complex viral metabolism in HCC progression, and diagnosing HBV patients predisposed to HCC (58). Rs4309483 polymorphism can also affect the expression of miRNA-122 and create a protection against CHB infection. However, it increases the risk of HCC in HBV carriers (59). Moreover, the basic expression of miRNA-122 can be highly influenced by rs2999200 and rs6551952 single nucleotide polymorphism (60).
MiRNA-122 and chemotherapy
Recently, the first miRNA mimic reached phase-one clinical studies and indicated the possibility of miRNA expression change in human liver (61). Overexpression of miRNA-122 is reported to increase the sensitivity of HCC cells to sorafenib (32). MiRNA-122 is also considered as a suppression of HCC tumor, which is resistance to doxorubicin (62). In this respect, a study showed that the enhanced expression of miR-122 in HepG2 cells, which are untreated by doxorubicin, could increase cellular sensitivity to chemotherapy drugs through down-regulation of MDR, ABCB1, and ABCF2 genes. Evaluation of cell cycle also indicated that the anti-proliferation effects of miRNA-122 could be associated with cell cycle arrest in G0/G1 phase. Moreover, miRNA-122 treatment and doxorubicin could increase the number of HCC cells in G0/G1 phase. The results revealed that the increase of miRNA-122 expression could arrest the cell cycle and inhibit the cellular growth of HCC which could be accompanied by down-regulation of MDR-related genes (63). In another study, the role of miRNA-122 in HCC metabolism was analyzed and the results showed that PKM2 could have a significant role in aerobic glycolysis. The miRNA-122-PKM2 pathway could be introduced as a new method for treating HCC (62). In this regard, SFR could be assumed as a lower sensor of vascular endothelial growth factor essential for the angiogenesis of VEGF endothelial cells. It should be noted that miRNA-122 can directly inhibit angiogenesis in vitro, and simultaneously inhibit ADAM10, SRF, and Igf1R genes (32).
Conclusions
Some of the miRNAs exclusive to HBV serum, such as miRNA-122, are likely to increase in the serum of HBV patients up to be 1.5-fold. Serum miR-122 levels can be highly correlated with HBsAg serum antigen indicating the quantity of HBV translation. Determining the correlated mechanism of HBsAg and miRNA-122 can be advantageous for understanding HBV viruses. In this respect, miRNA-122 can differentiate between HBV carriers with high and low risks of disease progression. Apart from the potential role of miRNAs in diagnosing and classifying patients, they can be a good method for evaluating treatment responses. They can also act as a drug. Potentially, expression level of miRNA-122 can be a suitable biomarker for evaluating treatment results in HBV patients (Table 1). In fact, apart from diagnostic functions, miRNA-122 can be a treatment target considering that miRNA-122 can have a significant role in the proliferation of hepatitis viruses. Treatment effects of miRNA-122 in hepatocytes are also so efficient so that they can prevent hepatocarcinogenesis. Increased use of transcript me sequences can also present an impartial method for miRNAs recognition. Thus, more studies are needed to create a new evaluation system for different diseases or prognosis of HBV infections using miRNA. While miRNA is endowed with a high potential as a biomarker for HBV, further investigations should be conducted on optimum miRNAs or other diagnostic methods. The potential limitation of studies can be the primary screening of the candidate miRNAs using microarray which typically contains only a limited set of probes that may not include recently-identified miRNAs nor adequately prevent cross-hybridization with unrepresented miRNAs. However, the tissue samples used in this study were small and inefficient, which were considered as a limitation. Accordingly, more research using multiple samples and functional tests were recommended to be carried out in order to confirm the validity of the given findings. Besides, experimental studies have indicated that miRNAs can have a significant role in the cell cycle of HBV, but more investigations should be conducted on miRNA-122 targeting the eradication of CCCDNA and viruses. The final objective should be creating a new HBV treatment method. According to the limited strategies used for preventing hepatic disease, HCC treatment, relationship between expression reduction of miRNA-122 and hepatic inflammation, fibrosis, steatosis (fatty change), and HCC; mimic miRNA-12 can be recognized as a functional method to reduce hepatic disease progression and improve HCC treatments. The future clinical trials using miRNA base molecules can thus present a bright perspective regarding hepatic disease treatment and HCC. Due to the all-encompassing role of miRNAs in hepatic homeostasis, cholesterol biosynthesis, and fatty acid metabolisms; a vast range of studies is also necessary for identifying an efficient dose of miRNAs in treatments. An additional preclinical investigation will be also required to conclude the optimal level of miRNA mimics in therapies and to evaluate the potential risks related to miRNA-122 overexpression or reduction. Modulating miRNAs in disease states, especially cancer, can also represent a promising frontier for pharmacotherapy that may be used in conjunction with classical chemotherapy to control the consequences of deregulated miRNA levels within cells. MiRNA-122 treatment can be advantageous in treating HBV/Leishmania patients, negative and positive HCC patients, and also prevent nonalcoholic steatohepatitis (NASH) and cirrhosis. However, there are a lot of challenges along the way of developing an efficient system for miRNA-122 mimic delivery to the body when they are supposed to be used in clinics. Moreover, optimizing the delivery path of miRNA, such as intra-portal or trans-arterial, is essential for successful miRNA-122 treatments of HCC patients. Developing new technologies for quantitative measurement of miRNA-122 in hepatic damages is also considered to be a major progress in diagnosis and prognosis of multiple hepatic diseases.
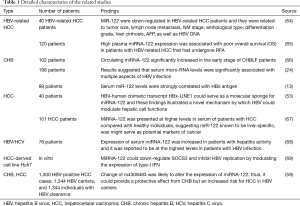
Full table
Acknowledgments
Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fields B, Knipe D, Howley P, et al. Fields virology. 5th. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2007.
- Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2:1129-33. [Crossref] [PubMed]
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45-55. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259-69. [Crossref] [PubMed]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 2006;11:441-50. [Crossref] [PubMed]
- Li LM, Hu ZB, Zhou ZX, et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res 2010;70:9798-807. [Crossref] [PubMed]
- Qi P, Cheng SQ, Wang H, et al. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One 2011;6:e28486. [Crossref] [PubMed]
- Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 2013;14:319. [Crossref] [PubMed]
- Bertino G, Ardiri AM, Calvagno GS, et al. Prognostic and diagnostic value of des-gamma-carboxy prothrombin in liver cancer. Drug News Perspect 2010;23:498-508. [Crossref] [PubMed]
- Anwar SL, Lehmann U. MicroRNAs: Emerging Novel Clinical Biomarkers for Hepatocellular Carcinomas. J Clin Med 2015;4:1631-50. [Crossref] [PubMed]
- Ji F, Yang B, Peng X, et al. Circulating microRNAs in hepatitis B virus-infected patients. J Viral Hepat 2011;18:e242-51. [Crossref] [PubMed]
- Waidmann O, Bihrer V, Pleli T, et al. Serum microRNA-122 levels in different groups of patients with chronic hepatitis B virus infection. J Viral Hepat 2012;19:e58-65. [Crossref] [PubMed]
- Zhang Y, Jia Y, Zheng R, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 2010;56:1830-8. [Crossref] [PubMed]
- Hayes CN, Akamatsu S, Tsuge M, et al. Hepatitis B virus-specific miRNAs and Argonaute2 play a role in the viral life cycle. PLoS One 2012;7:e47490. [Crossref] [PubMed]
- Luo X, Yang W, Ye DQ, et al. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet 2011;7:e1002128. [Crossref] [PubMed]
- Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol 2002;12:735-9. [Crossref] [PubMed]
- Girard M, Jacquemin E, Munnich A, et al. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol 2008;48:648-56. [Crossref] [PubMed]
- Moore KJ, Rayner KJ, Suarez Y, et al. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu Rev Nutr 2011;31:49-63. [Crossref] [PubMed]
- Waidmann O, Koberle V, Brunner F, et al. Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS One 2012;7:e45652. [Crossref] [PubMed]
- Qiu L, Fan H, Jin W, et al. miR-122-induced down-regulation of HO-1 negatively affects miR-122-mediated suppression of HBV. Biochem Biophys Res Commun 2010;398:771-7. [Crossref] [PubMed]
- Chen Y, Shen A, Rider PJ, et al. A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication. Faseb j 2011;25:4511-21. [Crossref] [PubMed]
- Wang S, Qiu L, Yan X, et al. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) -modulated P53 activity. Hepatology 2012;55:730-41. [Crossref] [PubMed]
- Arataki K, Hayes CN, Akamatsu S, et al. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. J Med Virol 2013;85:789-98. [Crossref] [PubMed]
- Li C, Wang Y, Wang S, et al. Hepatitis B virus mRNA-mediated miR-122 inhibition upregulates PTTG1-binding protein, which promotes hepatocellular carcinoma tumor growth and cell invasion. J Virol 2013;87:2193-205. [Crossref] [PubMed]
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011;108:5003-8. [Crossref] [PubMed]
- Hsu SH, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest 2012;122:2871-83. [Crossref] [PubMed]
- Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012;122:2884-97. [Crossref] [PubMed]
- Zeisel MB, Pfeffer S, Baumert TF. miR-122 acts as a tumor suppressor in hepatocarcinogenesis in vivo. J Hepatol 2013;58:821-3. [Crossref] [PubMed]
- Coulouarn C, Factor VM, Andersen JB, et al. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 2009;28:3526-36. [Crossref] [PubMed]
- Kutay H, Bai S, Datta J, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006;99:671-8. [Crossref] [PubMed]
- Bai S, Nasser MW, Wang B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem 2009;284:32015-27. [Crossref] [PubMed]
- Hsu SH, Yu B, Wang X, et al. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine 2013;9:1169-80. [Crossref] [PubMed]
- Coulouarn C, Corlu A, Glaise D, et al. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res 2012;72:2533-42. [Crossref] [PubMed]
- Fornari F, Gramantieri L, Giovannini C, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res 2009;69:5761-7. [Crossref] [PubMed]
- Xu Y, Xia F, Ma L, et al. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett 2011;310:160-9. [PubMed]
- Xu J, Zhu X, Wu L, et al. MicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/beta-catenin pathway. Liver Int 2012;32:752-60. [Crossref] [PubMed]
- Song K, Han C, Zhang J, et al. Epigenetic regulation of MicroRNA-122 by peroxisome proliferator activated receptor-gamma and hepatitis b virus X protein in hepatocellular carcinoma cells. Hepatology 2013;58:1681-92. [Crossref] [PubMed]
- Peng F, Xiao X, Jiang Y, et al. HBx down-regulated Gld2 plays a critical role in HBV-related dysregulation of miR-122. PLoS One 2014;9:e92998. [Crossref] [PubMed]
- Fan CG, Wang CM, Tian C, et al. miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3. Oncol Rep 2011;26:1281-6. [PubMed]
- Wen W, Ding J, Sun W, et al. Cyclin G1-mediated epithelial-mesenchymal transition via phosphoinositide 3-kinase/Akt signaling facilitates liver cancer progression. Hepatology 2012;55:1787-98. [Crossref] [PubMed]
- Wong CM, Ng IO. Molecular pathogenesis of hepatocellular carcinoma. Liver International 2008;28:160-74. [Crossref] [PubMed]
- Fatima S, Lee NP, Luk JM. Dickkopfs and Wnt/beta-catenin signalling in liver cancer. World J Clin Oncol 2011;2:311-25. [Crossref] [PubMed]
- Wang G, Zhao Y, Zheng Y. MiR-122/Wnt/beta-catenin regulatory circuitry sustains glioma progression. Tumour Biol 2014;35:8565-72. [Crossref] [PubMed]
- Zeng C, Wang R, Li D, et al. A novel GSK-3 beta-C/EBP alpha-miR-122-insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology 2010;52:1702-12. [Crossref] [PubMed]
- Wang SC, Lin XL, Li J, et al. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One 2014;9:e101330. [Crossref] [PubMed]
- Wu DB, Liu FW, Li J, et al. Intrahepatic IFN-alpha expression in liver specimens from HBV-infected patients with different outcomes. Eur Rev Med Pharmacol Sci 2013;17:2474-80. [PubMed]
- Koeberlein B, zur Hausen A, Bektas N, et al. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res 2010;148:51-9. [Crossref] [PubMed]
- Huang Y, Feld JJ, Sapp RK, et al. Defective hepatic response to interferon and activation of suppressor of cytokine signaling 3 in chronic hepatitis C. Gastroenterology 2007;132:733-44. [Crossref] [PubMed]
- Kim KA, Lin W, Tai AW, et al. Hepatic SOCS3 expression is strongly associated with non-response to therapy and race in HCV and HCV/HIV infection. J Hepatol 2009;50:705-11. [Crossref] [PubMed]
- Persico M, Capasso M, Russo R, et al. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut 2008;57:507-15. [Crossref] [PubMed]
- Shao RX, Zhang L, Peng LF, et al. Suppressor of cytokine signaling 3 suppresses hepatitis C virus replication in an mTOR-dependent manner. J Virol 2010;84:6060-9. [Crossref] [PubMed]
- Liang HW, Wang N, Wang Y, et al. Hepatitis B virus-human chimeric transcript HBx-LINE1 promotes hepatic injury via sequestering cellular microRNA-122. J Hepatol 2016;64:278-91. [Crossref] [PubMed]
- Zhang Q, Pu R, Du Y, et al. Non-coding RNAs in hepatitis B or C-associated hepatocellular carcinoma: potential diagnostic and prognostic markers and therapeutic targets. Cancer Lett 2012;321:1-12. [Crossref] [PubMed]
- Wang W, Zhao LJ, Tan YX, et al. Identification of deregulated miRNAs and their targets in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 2012;18:5442-53. [Crossref] [PubMed]
- Yu Z, Li Z, Jolicoeur N, et al. Aberrant allele frequencies of the SNPs located in microRNA target sites are potentially associated with human cancers. Nucleic Acids Res 2007;35:4535-41. [Crossref] [PubMed]
- Gao Y, He Y, Ding J, et al. An insertion/deletion polymorphism at miRNA-122-binding site in the interleukin-1alpha 3' untranslated region confers risk for hepatocellular carcinoma. Carcinogenesis 2009;30:2064-9. [Crossref] [PubMed]
- Du Y, Han X, Pu R, et al. Association of miRNA-122-binding site polymorphism at the interleukin-1 alpha gene and its interaction with hepatitis B virus mutations with hepatocellular carcinoma risk. Front Med 2014;8:217-26. [Crossref] [PubMed]
- Liu Y, Xie K, Wen J, et al. A genetic variant in microRNA-122 regulatory region confers risk for chronic hepatitis B virus infection and hepatocellular carcinoma in Han Chinese. J Med Virol 2014;86:1669-74. [Crossref] [PubMed]
- Gamazon ER, Innocenti F, Wei R, et al. A genome-wide integrative study of microRNAs in human liver. BMC Genomics 2013;14:395. [Crossref] [PubMed]
- Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget 2014;5:872-81. [Crossref] [PubMed]
- Pan C, Wang X, Shi K, et al. MiR-122 Reverses the Doxorubicin-Resistance in Hepatocellular Carcinoma Cells through Regulating the Tumor Metabolism. PLoS One 2016;11:e0152090. [Crossref] [PubMed]
- Yahya SMM, Fathy SA, El-Khayat ZA, et al. Possible Role of microRNA-122 in Modulating Multidrug Resistance of Hepatocellular Carcinoma. Indian J Clin Biochem 2018;33:21-30. [Crossref] [PubMed]
- Qiao DD, Yang J, Lei XF, et al. Expression of microRNA-122 and microRNA-22 in HBV-related liver cancer and the correlation with clinical features. Eur Rev Med Pharmacol Sci 2017;21:742-7. [PubMed]
- Cho HJ, Kim JK, Nam JS, et al. High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis B virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin Biochem 2015;48:1073-8. [Crossref] [PubMed]
- Wang WJ, Lai RT, Lu J, et al. Correlation between circulating miR-122 and prognosis of chronic HBV-related liver failure. J Dig Dis 2016;17:334-9. [Crossref] [PubMed]
- Xu J, Wu C, Che X, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 2011;50:136-42. [Crossref] [PubMed]
- Cheng HR, Kao JH, Wu HL, et al. Clinical significance of circulating miR-122 in patients with dual chronic hepatitis B and C virus infection. Hepatol Int 2015;9:35-42. [Crossref] [PubMed]
- Gao D, Zhai A, Qian J, et al. Down-regulation of suppressor of cytokine signaling 3 by miR-122 enhances interferon-mediated suppression of hepatitis B virus. Antiviral Res 2015;118:20-8. [Crossref] [PubMed]


