Patterns of care and outcomes of intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for anal cancer
Introduction
Definitive chemoradiation is the standard of care for anal squamous cell carcinoma (1,2). These trials utilized 3D-conformation radiation therapy (3DCRT) technique and a subsequent trial (RTOG 9811) evaluating chemoradiation with 5-FU/MMC versus 5-FU/cisplatin showed significant adverse events with 34% incidence of grade ≥3 GI toxicity and 48% grade ≥3 skin toxicity (3). Given that the majority of patients have curable disease, strategies to decrease long-term toxicity from treatment is an important area of focus.
While toxicity itself adds to the morbidity of treatment, studies have shown that treatment breaks due to toxicity are common in the management course and can adversely impact outcomes (4). One method to reduce treatment related toxicity is with IMRT, which has increasingly becoming the preferred technique over conventional radiation. NRG Oncology RTOG 0529 was a phase 2 study that showed improvement in hematologic, GI and skin toxicity profile with dose-painted IMRT over conventional radiation (5). The primary endpoint of improvement in adverse events by at least 15% was not met and 81% of patients on the study required re-planning on central review. To date, there has not been a randomized study evaluating the two radiation treatment modalities.
We sought to elucidate practice patterns in the U.S. and total radiation treatment times of IMRT versus 3D-conformal radiation therapy (3DCRT) for anal cancer using the National Cancer Database (NCDB).
Methods
The NCDB is a nationwide, hospital-based registry that consists of patients who received care at cancer centers accredited by the American College of Surgeons Commission on Cancer (CoC) and currently captures approximately 70% of all patients newly diagnosed with cancer. The CoC’s NCDB and the accredited facilities participating in the NCDB are the source of the de-identified data used in this study. However, they have not verified and are not responsible for the statistical validity or conclusions derived by the authors of this study. Exemption was obtained from the New York Harbor Veterans Affairs Committee for Research and Development prior to the initiation of this study.
The NCDB was queried for patients with non-metastatic squamous cell carcinoma of the anus from 2004–2013 who received definitive chemoradiation. Concurrent chemoradiation was defined as receipt of either chemotherapy or radiation within 14 days of each other. The cohort was further selected for those who received either IMRT or 3DCRT and total radiation dose received was limited to 4,500–6,000 cGy. To account for immortal time bias, patients living less than 6 months from the time of diagnosis were excluded (6). Those who received RT outside of the primary area were also excluded.
The primary goal of this analysis was to assess patterns of care regarding IMRT use over time. The secondary goal was to analyze survival. Patient-related factors included age, race (White, Black, Other), gender (male, female), Charlson-Deyo comorbidity index (0, 1, ≥2), insurance type (not insured, private, Medicaid, Medicare, other/unknown), and median income quartile. Clinical-related factors included primary tumor size (<2, 2–5, >5 cm), clinical node status (negative/unknown, positive), HPV status (negative, positive), facility type (non-academic, academic), region of treatment (Northeast, Midwest, South, West) and treatment year. Total radiation treatment time was defined as the number of days from the start to the end of radiation therapy and was stratified by those treated for <7 and ≥7 weeks.
Statistical analysis
Patient- and clinical-related factors were compared via the Chi-square and Mann-Whitney tests when appropriate between those treated with IMRT versus 3DCRT. Univariable logistic regression was performed to assess for predictors of IMRT usage. The variables included age (<50, 50–60, >60 years), total radiation treatment time (<7, ≥7 weeks), race (White, Black, Other), gender (male, female), Charlson-Deyo comorbidity index (0, 1, ≥2), primary tumor size (<2, 2–5, >5 cm), clinical node status (negative/unknown, positive), facility type (non-academic, academic), insurance status (not insured, private insurance, Medicaid, Medicare, other/unknown), median income quartile and years of diagnosis (2004–2006, 2007–2010, 2011–2013). Variables with a P value <0.10 on univariable analysis were planned to be included in the multivariable analysis.
Overall survival curves comparing 3DCRT and IMRT were generated using the Kaplan-Meier method and compared via the log-rank test. Univariable and multivariable Cox regression was used to determine covariables associated with differences in overall survival. Factors associated with a P value <0.10 on univariable analysis were included in the multivariable analysis. To assess whether treatment modality and total radiation treatment time potentially confounded one another, an interaction term was created and was analyzed in the regression model. All analysis was performed using SPSS version 21.0 (IBM Inc, Armonk, NY, USA).
Results
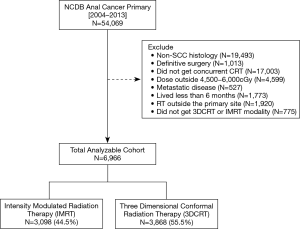
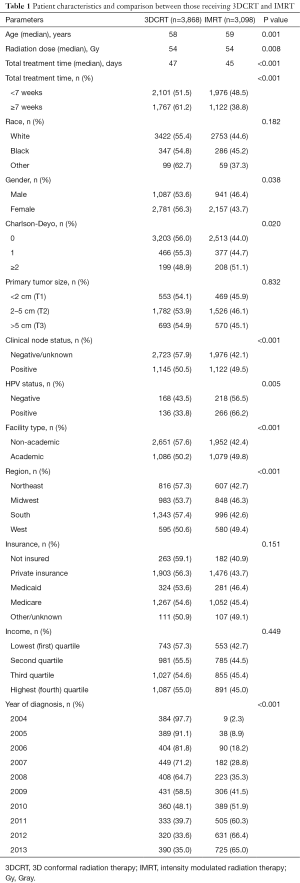
There were 6,966 patients who met the study criteria, of which 3,868 (55.5%) received 3DCRT and 3,098 (44.5%) received IMRT (Figure 1). Median follow up was 40.7 months and median RT dose was 5,400 cGy (IQR, 5,040–5,580 cGy) for the entire cohort. Median total radiation treatment time was 47 days (IQR, 43–57 days) for 3DCRT and 45 days (IQR, 42–52 days) for IMRT (P<0.001). The utilization of IMRT increased from 2.3% in 2004 to 65% in 2013. Total radiation treatment time was <7 weeks for 54.3% of patients treated with 3DCRT versus 63.8% of patients treated with IMRT. Further details regarding patient and clinical characteristics between the two treatment groups can be found in Table 1.
Full table
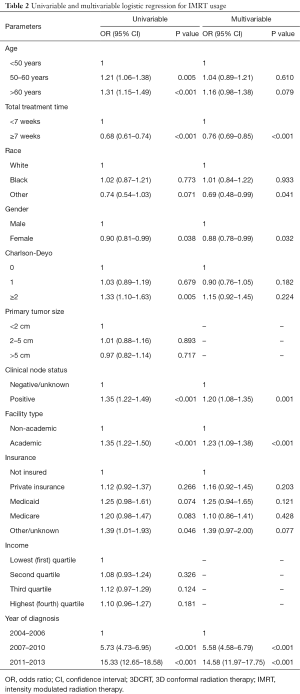
On multivariable logistic regression, positive clinical nodes (OR =1.20; 95% CI, 1.08–1.35; P=0.001), treatment at an academic facility (OR =1.23; 95% CI, 1.09–1.38; P<0.001) and more recent year of diagnosis (OR 5.58–14.58; P<0.001) were associated with increased likelihood of receiving IMRT. Total radiation treatment time ≥7 weeks (OR =0.76; 95% CI, 0.69–0.85; P<0.001) and female gender (OR =0.88; 95% CI, 0.78–0.99; P=0.032) were associated with decreased likelihood of receiving IMRT. Further details can be found in Table 2.
Full table
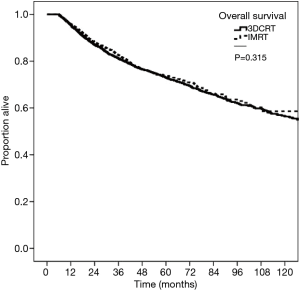
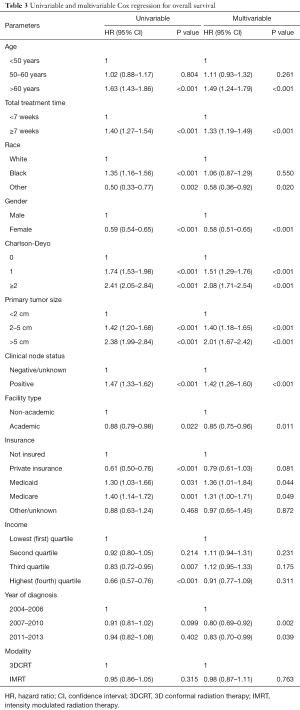
Kaplan-Meier curves depicting survival in patients grouped by RT modality of 3DCRT versus IMRT are shown in Figure 2. The 5-year OS was 73.0% for 3DCRT and 73.9% for IMRT (P=0.315). On multivariable survival analysis, age >60 years (HR =1.49; 95% CI, 1.24–1.79; P<0.001), total radiation treatment time ≥7 weeks (HR =1.33; 95% CI, 1.19–1.49; P<0.001), Charlson-Deyo score >0 (HR 1.51–2.08, P<0.001), and tumor size >2 cm (HR =1.40–2.01; P<0.001) were associated with worse survival. Female gender (HR =0.58; 95% CI, 0.51–0.65; P<0.001), treatment at an academic facility (HR =0.85; 95% CI, 0.75–0.95; P=0.011) and more recent years of diagnosis (HR =0.80–0.83, P<0.05) were associated with improved survival. Radiation modality (3DCRT vs. IMRT) did not impact survival (HR =0.98; 95% CI, 0.87–1.11; P=0.763). Additional Cox proportional hazard submodels did not detect a significant interaction effect between mode of RT and increasing treatment time. Summary of the univariate and multivariate models can be found in Table 3.
Full table
Discussion
In this large hospital-based cohort, IMRT use had increased from 2% to 65% over the study period (P<0.001). On multivariable Cox regression model, treatment modality did not impact survival and the two groups were not significantly different on Kaplan-Meier analysis. However, IMRT was less likely than 3DCRT to have prolonged radiation treatment duration, which was associated with worse survival.
The benefit of IMRT has been clearly established in both prospective (5,7) and retrospective studies (8-11) by its ability to provide conformality of the dose to the target and spare normal structures while maintaining local control. Studies directly comparing IMRT to 3DCRT have consistently shown improvements in toxicity while some have even shown a benefit to overall survival.
A retrospective study from Stanford of anal cancer patients treated with chemotherapy and 3DCRT (n=17) versus IMRT (n=29) found 65% of patients in the 3DCRT had grade >2 nonhematologic toxicity compared to 21% in the IMRT group (P=0.003) (12). The IMRT group also showed benefit at 3 years for OS, LRC and PFS over 3DCRT (P<0.01) however, these latter findings have not been reproduced in other retrospective studies. A larger retrospective review at Memorial Sloan Kettering found that the 45 patients treated with IMRT had significantly higher N-stage (P<0.01) than the 178 patients who received 3DCRT but there was no difference in recurrence-free survival, metastases-free survival and overall survival at 2 years, even after propensity score matching (13). The present study similarly found that patients treated with IMRT were more likely to have node positive disease (OR =1.20, P=0.001). A retrospective UK study of 10 patients comparing dosimetric coverage of both IMRT and 3DCRT found that IMRT significantly reduced dose to organs at risk while maintaining excellent PTV coverage (14) thus, careful target delineation with modern CT-based techniques may allow adequate coverage to high-risk regions.
Arguably the most notable finding of the current study was that patients treated with IMRT were less likely to have a total radiation treatment time over 7 weeks (OR =0.76, P<0.001) and those who had longer total treatment times had worse survival (HR =1.33, P<0.001). While the NCDB does not code for data regarding toxicity or reason for prolonged treatment times, this is likely due to chemoradiation-related toxicity as these events have been reported to occur in up to 80% of anal cancer patients (15).
Multiple studies have now shown that prolonged treatment times and interruptions are associated with poorer outcomes (12,15,16), which is supported by our study. Bazan et al. found that those in the 3DCRT group had a median treatment duration of 57 days compared to 40 days for the IMRT group and the latter had significant improvements in survival. Another retrospective study by Huang et al. found that among 28 consecutive patients treated with dose-escalated chemoradiation, longer treatment breaks was associated with a higher local failure rate even after accounting for higher local dose. Specifically, those who received more than 54 Gy within 60 days had 2-year local PFS of 89% compared to 42% (P=0.01) for those who received more less than 54 Gy or longer than 60 days.
Yet treatment time has been carefully examined in a pooled analysis of 937 patients from RTOG 98-04 and RTOG 98-11. This investigation showed no correlation with duration of radiation therapy and local control. This was also the metric used in the present study but prolonged total treatment time, which includes the utilization of neoadjuvant chemotherapy, was associated with higher local failure (HR =1.52; P=0.005) and colostomy rates (HR =1.51; P=0.02) (4).
The management of undue side effects was evaluated in a 2014 linked SEER-Medicare database showing unplanned health care utilization costs such as emergency department visits and hospitalizations were higher among patients receiving 3DCRT over IMRT (median, $4,957 vs. $711; P=0.02) however, IMRT was associated with higher total costs than 3DCRT as expected (median total cost $35,890 vs. $27,262; P<0.001) (17). In the present study, income quartile and insurance status were not associated with increased utilization of IMRT, which may indicate high acceptance rates of insurance companies of IMRT.
In the present study, we found the utilization of IMRT was associated with academic centers (OR =1.23; P<0.001) as well as more recent years of diagnosis (OR 5.58–14.58; P<0.001). Academic centers may be more likely to adopt new technologies or at least incorporate them into clinical trial settings. These findings were similarly found in another NCDB analysis examining patterns of care of these two modalities in anal cancer (18). While the prior NCDB study focused on disparities and utilization of IMRT, the current analysis includes radiation treatment duration with a more stringent selection criteria that excludes potential confounders such as immortal time bias and dosing levels that may indicate palliative intent.
There are limitations to this study as is with any hospital-based database. We did not have data regarding the type of chemotherapeutic agent used and why some patients had longer radiation treatment time than others. Furthermore, we did not have data regarding smoking status and HIV status, as these covariates may have impacted outcomes (19). Most importantly, there was no data regarding toxicity thus this important endpoint could not be evaluated.
Conclusions
IMRT has dramatically increased in utilization from 2% to 65% during the study time period. There were no survival differences between 3DCRT and IMRT. However, IMRT was less likely than 3DCRT to have prolonged treatment times, which was associated with worse survival.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Exemption was obtained from the New York Harbor Veterans Affairs Committee for Research and Development prior to the initiation of this study.
References
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. [Crossref] [PubMed]
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. [Crossref] [PubMed]
- Ben-Josef E, Moughan J, Ajani JA, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol 2010;28:5061-6. [Crossref] [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1365-73. [Crossref] [PubMed]
- Han K, Cummings BJ, Lindsay P, et al. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys 2014;90:587-94. [Crossref] [PubMed]
- Pepek JM, Willett CG, Wu QJ, et al. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys 2010;78:1413-9. [Crossref] [PubMed]
- Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007;25:4581-6. [Crossref] [PubMed]
- Kachnic LA, Tsai HK, Coen JJ, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012;82:153-8. [Crossref] [PubMed]
- Arcadipane F, Franco P, Ceccarelli M, et al. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia Pac J Clin Oncol 2018;14:217-23. [Crossref] [PubMed]
- Bazan JG, Hara W, Hsu A, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011;117:3342-51. [Crossref] [PubMed]
- Dasgupta T, Rothenstein D, Chou JF, et al. Intensity-modulated radiotherapy vs. conventional radiotherapy in the treatment of anal squamous cell carcinoma: a propensity score analysis. Radiother Oncol 2013;107:189-94. [Crossref] [PubMed]
- Brooks CJ, Lee YK, Aitken K, et al. Organ-sparing Intensity-modulated radiotherapy for anal cancer using the ACTII schedule: a comparison of conventional and intensity-modulated radiotherapy plans. Clin Oncol (R Coll Radiol) 2013;25:155-61. [Crossref] [PubMed]
- Roohipour R, Patil S, Goodman KA, et al. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum 2008;51:147-53. [Crossref] [PubMed]
- Huang K, Haas-Kogan D, Weinberg V, et al. Higher radiation dose with a shorter treatment duration improves outcome for locally advanced carcinoma of anal canal. World J Gastroenterol 2007;13:895-900. [Crossref] [PubMed]
- Chin AL, Pollom EL, Qian Y, et al. Impact of Intensity-Modulated Radiotherapy on Health Care Costs of Patients With Anal Squamous Cell Carcinoma. J Oncol Pract 2017;13:e992-1001. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Utilization of intensity modulated radiation therapy for anal cancer in the United States. J Gastrointest Oncol 2018;9:466-77. [Crossref] [PubMed]
- Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum 2001;44:1496-502. [Crossref] [PubMed]