
The efficacy of everolimus and sunitinib in patients with sporadic or germline mutated metastatic pancreatic neuroendocrine tumors
Introduction
Neuroendocrine tumors (NETs) account for less than 1% of all human cancer cases, affecting about 6 individuals per 100,000 per year in Europe (1). NETs have been reported in almost all organs of the body because they derive from neuroendocrine stem cells, distributed during embryogenesis throughout the neural crest, endocrine glands and diffuse endocrine system. Although they are mostly sporadic, approximately 10% can occur as part of hereditary syndromes, mainly multiple endocrine neoplasia type 1 (MEN1) and Von-Hippel Lindau syndrome (VHL) (2).
The MEN1 syndrome is autosomal dominant, with a prevalence of 2–3 per 100,000, being one of the most common familial cancer syndromes (3). While 30–80% of MEN1 patients develop pancreatic NETs (pNETs), other commonly associated tumors are adenomas of the pituitary gland and duodenum gastrinomas (3-5). In MEN1 patients, pNETs are often nonfunctional (3,4). Importantly, pNETs are the main cause of death among patients with MEN1, with a specific disease survival rate at 10 years ranging between 23% and 62% (6-8). In patients with VHL syndrome, pNETs develop in 10% to 17% and are almost exclusively nonfunctional (9-11). In addition to pancreatic neoplasms, VHL patients often develop a variety of benign and malignant neoplasms, including clear cell renal carcinomas, pheochromocytomas, paragangliomas, hemangioblastomas, retinal angiomas, middle ear endolymphatic sac tumors and papillary cystadenomas of the epididymis and the broad ligament (12,13).
These cancer predisposition syndromes are characterized by deleterious germline mutations in genes that increase tumor susceptibility in the pancreas and other neuroendocrine organs, leading to the development of multiple tumors. However, in sporadic pNETs, the genetic changes involved in neuroendocrine tumorigenesis in the pancreas have just started to be elucidated. Jiao et al. performed complete exome sequencing in a set of 10 cases of sporadic pNETs (primary tumor samples) and subsequently the most commonly mutated genes were analyzed in a validation set of 58 pNETs samples. Interestingly, they found that 44% of the tumors had somatic inactivation mutations in MEN1, 43% in ATRX and mutations in genes encoding proteins in the mTOR pathway were found in 14% of cases (14). Given that mutations in MEN1 lead to mTOR hyperactivation (15), it is intuitive to think that mTOR inhibition would be particularly effective in patients with MEN1 syndrome (16). Likewise, there is a rationale to infer that sunitinib, an antiangiogenic agent, would be also beneficial to patients with VHL-associated pNETs. However, to our knowledge, NET patients with such genetic syndromes have been under represented in pivotal phase III trials of sunitinib or everolimus. Therefore, aimed to evaluate the efficacy results of everolimus and sunitinib in patients with pNETs with and without MEN1 or VHL syndromes.
Methods
Study design
We conducted a multicenter retrospective and comparative study to assess the efficacy of everolimus and/or sunitinib in a cohort of patients with advanced pNETs with or without known MEN1 or VHL syndrome. Eligible patients had histological diagnosis of well differentiated NET of pancreatic origin, locally advanced unresectable or metastatic disease and received at least one month of sunitinib or everolimus in monotherapy. The evaluation of the germline mutational status of VHL and MEN1 was retrospectively collected from the medical records and their testing followed standard guidelines for screening based on clinical suspicion. All pNET patients were consecutively selected through an administrative list from one center (A.C. Camargo Cancer Center, Sao Paulo, Brazil) from September 2009 to April 2018; because of its rarity, we also included patients with a genetic diagnosis of VHL- and MEN1-associated metastatic pNET from other three centers in Brazil. The following clinical data were collected: sex, age at onset of targeted therapy, cell differentiation, ki-67 index, metastatic sites, and treatment outcomes.
The study was conducted in accordance with the protocol, Good Clinical Practice guidelines of the International Conference on Harmonization (ICH GCP) and applicable local laws and regulatory requirements, and was approved by the Research Ethical Committees.
The primary endpoints were progression free survival (PFS) and time to treatment failure (TTF) of each group. PFS was defined as the time between the first dose of everolimus/sunitinib and discontinuation for disease progression or death. Progression was defined as per the oncologists evaluation as documented in the medical charts. TTF was defined as the time between the first dose of everolimus/sunitinib and discontinuation for any reason, including disease progression, treatment toxicity, patient preference or death. If no event has occurred, the date of the last evaluation was censored.
Descriptive statistics techniques were used to calculate measures such as mean, median and standard deviations of the demographic characteristics of the studied population. The t-test of independent samples for continuous variables and Pearson’s Chi-square test or Fisher’s Exact for categorical variables were used for comparisons between group variables (pNET germline mutated and sporadic). The Kaplan-Meier method was used to calculate the survival curves and they were compared using the log-rank test. For all analyzes, we considered the level of statistical significance of 5% (P value of baseline <0.05). In view of the rarity of the disease under study, the sample size was convenience-based, without a formal calculation.
Results
From September 2009 to April 2018, 33 patients were identified. Table 1 summarizes the characteristics of the patients. Their median age was 51 years (range, 24–83 years), and the female/male ratio was 39.4%/60.6%. The median Ki-67 was 9% (range, 1–70%), 32 (97.0%) presented liver metastases and 7 (21.2%) patients had bone metastases. The majority of tumors were non-functioning
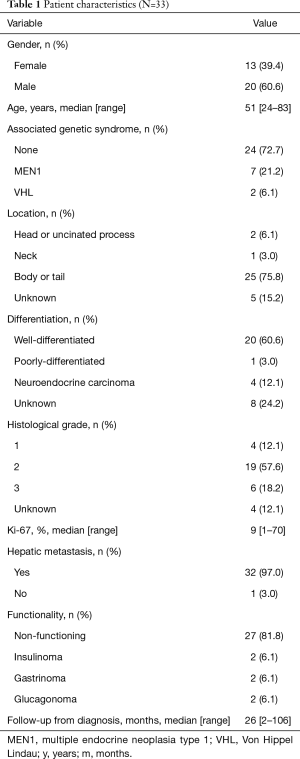
Full table
Out of 33 patients, 31 received everolimus, mostly in first and second lines (38.7% and 25.8%, respectively), of them, 23 were sporadic and 8 had germline mutations (6 in MEN1 and 2 in VHL genes). Nine patients received sunitinib—7 patients received both drugs. Out of the 9 patients that received sunitinib, 6 were sporadic and 3 had germline mutations (2 in MEN1 and 1 in VHL genes).
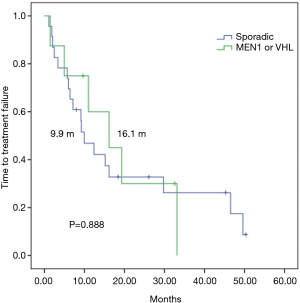
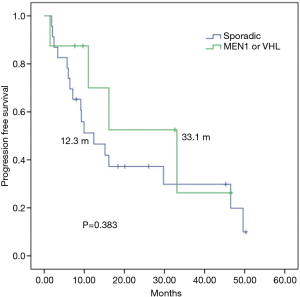
In a median follow up of 26 months, among 31 everolimus-treated patients, the median TTF (mTTF) and median PFS (mPFS) from initiation of everolimus was 12.3 and 15.1 months respectively. The mTTF was numerically superior in patients with germline mutations in MEN1 or VHL compared with those with sporadic pNETs (16.1 vs. 9.9 months respectively; P=0.888) (Figure 1). The mPFS was also numerically superior among germline mutated pNETs (33.1 vs. 12.3 months respectively; P=0.383) (Figure 2). Patients receiving everolimus in first line showed numerically better mTTF compared with later lines (16.1 vs. 9.2 months respectively; P=0.392); this was also observed in mPFS (33.1 vs. 11 months respectively; P=0.138).
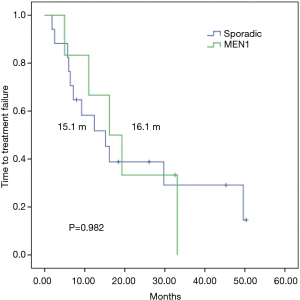
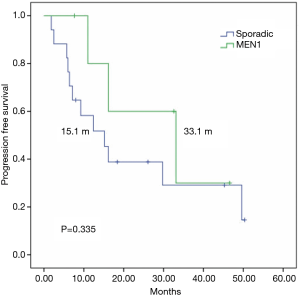
To control for imbalances between the groups and to analyze a more homogenous sample, performed a subgroup analysis of patients with grade 1–2 sporadic or MEN1-associated pNETs, since G3 tumors in the sporadic group could lead to worse outcomes in this group, The mTTF was similar in patients with or without MEN-1 syndrome (16.1 vs. 15.1 months respectively; P=0.982) (Figure 3), whereas mPFS was numerically superior among patients with MEN1 syndrome (33.1 vs. 15.1 months respectively; P=0.335) (Figure 4).
Disease control with everolimus [DC = complete response (CR) + partial response (PR) + stable disease (SD)] was observed in 74.1%, whereas 25.9% had upfront progressive disease (PD). If we separate these findings by sporadic vs. germline mutated pNETs, we obtained DC in 68.4% vs. 87.5% and upfront PD in 31.6% vs. 12.5%, respectively. The reasons for interruption were PD in 20 patients (83.3%), adverse events in 3 patients (12.5%); and others (physician’s choice) in 1 patient (4.2%).
Sunitinib was used by one patient with VHL syndrome in second line, achieving a PFS of 17.6 months. Other two patients with MEN1 syndrome used sunitinib with PFS of 1.3 and 2.9 months in second and fifth line, respectively. In the subgroup of sporadic pNETs, sunitinib was used by 6 patients reaching a mPFS of 18 months (range, 5–25 months), predominantly in second line.
Discussion
Although not statistically significant, we observed prolonged mPFS and mTTF, and superior DC in patients with MEN1 or VHL-associated pNETs treated with everolimus. The number of patients who received sunitinib was too small to reach any conclusion.
Studies show that approximately 80% of non-functional pNETs are detected when the disease is already unresectable, often with liver metastases. The 5-year survival rate of these patients is 30% to 40%, with median survival up to 44 months (17-20). One of the few case reports in the literature described a patient with pNET and genetically confirmed MEN1 syndrome who achieved 3 years of disease control with everolimus (21). Our cohort showed a numerically, albeit not statistically significant, superior mPFS and mTTF in the germline mutated group treated with everolimus, achieving a mPFS of 33 months, which seems longer than the mPFS of 12 months reported in the phase III placebo-controlled trials of everolimus (22). Compared with placebo, everolimus use was associated with a significant increase in the median PFS (11.0 vs. 4.6 months, P<0.0001) and sub-analyzes in the same cohort of patients confirmed their efficacy in all subgroups considered (prior chemotherapy, previous therapy with somatostatin analogues, performance status, age, sex, origin, well differentiated vs. intermediate grade tumors) (22). While the encouraging results of the mutated patients could be associated with selection bias from our small sample, the mPFS of the sporadic pNET group was quite similar—15 months—to that described in Radiant-3 trial.
Everolimus acts as a mammalian target of rapamycin (mTOR) inhibitor. The mTOR is a key protein implicated in the pathogenesis of many types of cancer, including NETs (23). The mTOR pathway regulates growth, proliferation, metabolism, angiogenesis and apoptosis, among other critical cell functions in response to multiple signals (24). The germinal inactivation of the MEN1 gene, when biallelic, leads to the formation of several types of NETs (14,25). Menin, the product of the MEN1 gene, suppresses proliferation through the mTOR pathway, inhibiting the activity of serine-threonine kinase AKT1 (15). Thus, inactivating mutations in MEN1 gene result in activation of AKT1 and, therefore, growth and proliferation. Everolimus is a standard and effective treatment in gastroenteropancreatic NETs (26) and could be particularly effective in MEN1 associated pNETs as seen in our study.
In the case of the VHL syndrome, the germline mutation of VHL, a tumor suppressor gene located in chromosome 3 (3p25-26) (27), leads to the development of pNETs by mechanisms not completely understood. It is known that the mutant VHL protein results in a lack of degradation of hypoxia-inducible factors (HIF) and ultimately in an uncontrolled production of factors that promote angiogenesis and tumor growth (13). In normal tissue, HIF-1α undergoes ubiquitination by the VHL protein, leading to the degradation of HIF-1α (28). Under hypoxia conditions (or VHL syndrome), HIF-1α is not degraded and accumulates in the nucleus, leading to an increased transcription of numerous hypoxia response genes [such as VEGF and carbonic anhydrase IX (Ca-IX)] (29,30). In addition, the mTOR pathway is also involved in the regulation of HIF-1α activity (31), this explains why human cancers with aberrant mTOR signaling are prone to angiogenesis and suggests that inhibition of mTOR with everolimus might be a suitable therapeutic strategy in patients with VHL syndrome.
Considering these characteristics, another well-established treatment for pNET is the multi-tyrosine kinase inhibitor (TKI) sunitinib, because of its anti-angiogenic mechanism of action. The phase III placebo-controlled trial of sunitinib in pNETs showed that the mPFS in the treatment group was more than doubled that of the control group, demonstrating the efficacy of this therapeutic approach (32,33). This is also true for pazopanib—a similar TKI, in patients with VHL syndrome (34). Although only one patient with VHL syndrome used sunitinib in our study, the time of disease control was superior to those reported in the literature.
The retrospective nature of this study is a limitation, as well as the reduced size of the population, and the use of convenience sampling when selecting the germline mutated patients. However, given the rarity if the genetic syndromes-associated pNETs, our study brings new data on outcomes of targeted therapy, particularly everolimus, to molecularly defined NETs.
In conclusion, our study suggests that everolimus offers a prolonged tumor control in patients with MEN1 or VHL-associated pNETs. Because of the small number of patients treated with sunitinib, we cannot conclude whether its efficacy is more pronounced among VHL patients. This study sheds light on an unexplored topic and supports the hypothesis that targeted directed treatment is the future for therapy of germline- (and even sporadic) mutated pNETs.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the protocol, Good Clinical Practice guidelines of the International Conference on Harmonization (ICH GCP) and applicable local laws and regulatory requirements, and was approved by the Comite de Etica em Pesquisa em Seres Humanos (CEP) of the Fundacao Antonio Prudente - AC Camargo Cancer Center (2632/18).
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Cives M, Strosberg J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology (Williston Park) 2014;28:749-56, 758. [PubMed]
- Marx S, Spiegel AM, Skarulis MC, et al. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med 1998;129:484-94. [Crossref] [PubMed]
- Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: Advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008;113:1807-43. [Crossref] [PubMed]
- Pannett AA, Thakker RV. Multiple endocrine neoplasia type 1. Endocr Relat Cancer 1999;6:449-73. [Crossref] [PubMed]
- Kouvaraki MA, Shapiro SE, Cote GJ, et al. Management of Pancreatic Endocrine Tumors in Multiple Endocrine Neoplasia Type 1. World J Surg 2006;30:643-53. [Crossref] [PubMed]
- Goudet P, Murat A, Binquet C, et al. Risk factors and causes of death in men1 disease. a gte (groupe d’etude des tumeurs endocrines) cohort study among 758 patients. World J Surg 2010;34:249-55. [Crossref] [PubMed]
- Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 2006;243:265-72. [Crossref] [PubMed]
- Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. Gastroenterology 2000;119:1087-95. [Crossref] [PubMed]
- Lubensky IA, Pack S, Ault D, et al. Multiple Neuroendocrine Tumors of the Pancreas in von Hippel-Lindau Disease Patients Histopathological and Molecular Genetic Analysis. Am J Pathol 1998;153:223-31. [Crossref] [PubMed]
- Schmitt AM, Schmid S, Rudolph T, et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer 2009;16:1219-27. [Crossref] [PubMed]
- Öberg K. The Genetics of Neuroendocrine Tumors. Semin Oncol 2013;40:37-44. [Crossref] [PubMed]
- Woodward ER, Maher ER. Von Hippel-Lindau disease and endocrine tumour susceptibility. Endocr Relat Cancer 2006;13:415-25. [Crossref] [PubMed]
- Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. [Crossref] [PubMed]
- Wang Y, Ozawa A, Zaman S, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res 2011;71:371-82. [Crossref] [PubMed]
- Neychev V, Steinberg SM, Cottle-Delisle C, et al. Mutation-targeted therapy with sunitinib or everolimus in patients with advanced low-grade or intermediategrade neuroendocrine tumours of the gastrointestinal tract and pancreas with or without cytoreductive surgery: Protocol for a phase II clinical trial. BMJ Open 2015;5:e008248. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The Epidemiology of Gastroenteropancreatic Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Khasraw M, Gill A, Harrington T, et al. Management of advanced neuroendocrine tumors with hepatic metastasis. J Clin Gastroenterol 2009;43:838-47. [Crossref] [PubMed]
- Frilling A, Sotiropoulos GC, Li J, et al. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361-79. [Crossref] [PubMed]
- Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. J Clin Oncol 2016;34:3906-13. [Crossref] [PubMed]
- Maia MC, Muniz Lourenço D Jr, Riechelmann R. Efficacy and Long-Term Safety of Everolimus in Pancreatic Neuroendocrine Tumor Associated with Multiple Endocrine Neoplasia Type I: Case Report. Oncol Res Treat 2016;39:643-5. [Crossref] [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N Engl J Med 2011;364:514-23. [Crossref] [PubMed]
- Kasajima A, Pavel M, Darb-Esfahani S, et al. mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2011;18:181-92. [Crossref] [PubMed]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 2006;5:671-88. [Crossref] [PubMed]
- Corbo V, Dalai I, Scardoni M, et al. MEN1 in pancreatic endocrine tumors: Analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocr Relat Cancer 2010;17:771-83. [Crossref] [PubMed]
- Riechelmann RP, Weschenfelder RF, Costa FP, et al. Guidelines for the management of neuroendocrine tumours by the Brazilian gastrointestinal tumour group. Ecancermedicalscience 2017;11:716. [Crossref] [PubMed]
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993;260:1317-20. [Crossref] [PubMed]
- Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res 2001;21:4317-24. [PubMed]
- Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 2002;8:2595-604. [PubMed]
- Speisky D, Duces A, Bièche I, et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and von Hippel-Lindau patients. Clin Cancer Res 2012;18:2838-49. [Crossref] [PubMed]
- Land SC, Tee AR. Hypoxia-inducible factor 1α is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 2007;282:20534-43. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Vinik AI, Raymond E. Pancreatic neuroendocrine tumors: Approach to treatment with focus on sunitinib. Therap Adv Gastroenterol 2013;6:396-411. [Crossref] [PubMed]
- Jonasch E, McCutcheon IE, Gombos DS, et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol 2018;19:1351-9. [Crossref] [PubMed]


