
Pelvic exenteration for locally advanced and recurrent rectal cancer—how much more?
Introduction
The management of patients with locally advanced or recurrent rectal cancer has evolved dramatically in recent decades (1). Although total pelvic exenteration (PE) was originally performed in the 1940s as a palliative procedure in an attempt to improve the quality of life of patients with advanced cervical cancer, it now represents the treatment of choice for patients with advanced or recurrent rectal cancer, and the only potentially curative option in a group who would otherwise be palliated. While rates of postoperative morbidity and mortality were initially high, surgical technique and patient selection has been refined, and imaging and radiation technology has advanced. As a result, PE is now performed routinely at specialised centres, offering patients a chance of long-term survival with acceptable morbidity and quality of life (2-6).
Clear (R0) resection margins has been demonstrated to be the most important factor in predicting both long-term survival and postoperative quality of life, and therefore achieving R0 resection with acceptable morbidity has become the ultimate goal of curative exenterative surgery (7,8). In recent decades, a number of surgical techniques have been developed in order to allow en bloc resection of ‘higher and wider’ tumours beyond the traditional mesorectal planes (9), including high sacrectomy, pubic bone resection and lateral compartment excision involving major neurovascular structures. In the most recent literature, R0 resection has been achieved in 55–80% of patients with recurrent rectal cancer, which translates to 5-year overall survival of 28–50% (2,5,6). This article explores the development of these radical techniques, current outcomes and future directions in exenteration surgery.
Historical context
Until the 1940s, advanced cervical cancer was considered beyond the scope of curative treatment. Women with advanced disease commonly died after long periods with intractable pain, intestinal or ureteric obstruction, and almost half did so without metastatic disease (10). PE was first described by Alexander Brunschwig as an ablative procedure for palliation (11). The first description of PE in a patient with locally advanced rectal cancer was by Thompson and Howe in 1950 (12). Survival outcomes from these early publications were modest at best with mortality rates reported up to 23% (1,11). Over the following decades, due to advancements in anaesthetics and perioperative medical care, surgical technique and imaging, PE evolved into a potentially curative treatment with a reasonable quality of life (1).
Evolution of the Royal Prince Alfred Hospital Pelvic Exenteration Unit
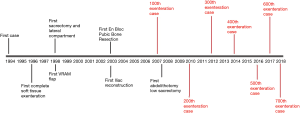
The progression and evolution of PE in our unit followed a similar pathway. The first decade was mainly focused on the safety and collaboration with other centres to improve outcomes and ensuring safety of PE. With the success built on central compartment exenteration, development of novel techniques ensued with a focus on ensuring negative resection margins in the second decade (13). Multiple publications and meta-analysis have emphasized the importance R0 resection (7) and its influence on survival. This has encouraged more exenterative surgeons to go further beyond the traditional total mesorectal excision plane in the pursue of negative margins. Functional outcomes and quality of life will be the next focus as resection goes higher and wider. Figure 1 shows the key developmental milestones achieved in our unit.
Posterior compartment
Despite initial attempts reported in the 1960s by Brunschwig, PE with composite sacrectomy was not really developed until the 1980s due to such poor morbidity and mortality outcomes (14,15). Like in other compartments, the most important factor when pursuing curative resections in the posterior pelvis is complete oncological resection. For tumours that abut or infiltrate the presacral fascia, en bloc sacrectomy should be performed rather than attempting to ‘shave’ the fascia from the sacral bone which may lead to microscopically involved margins. If the level of sacral transection is below the level of the sacroiliac joint (below S3), the sacrectomy is performed trans-abdominally using an osteotome, referred to as abdominolithotomy sacrectomy (16). This gives better access to the pelvic sidewall and control of the iliac vasculature and permits a more lateral dissection of the lumbosacral trunk and sacral nerve roots as they traverse lateral to the ischial spine via the greater sciatic foramen to form the sciatic nerve. For more proximal sacral bone involvement the patient is turned prone after the abdominoperineal phase for traditional prone sacrectomy (17). A recent systematic review reported 2% mortality, 52% major morbidity and 78% R0 resection in 220 patients who underwent sacrectomy as part of salvage surgery for locally recurrent rectal cancer (18). Median overall survival in patients with R0 resection was up to 34 months.
High sacrectomy has been performed with outcomes comparable to that of low sacrectomy at specialised units and in selected patients (17,19,20). In our unit’s experience with en bloc sacrectomy for locally recurrent rectal cancer, an R0 rate of 74% was achieved which conveyed an overall 5-year survival of 38%, with major complications occurring in 39% of patients and no perioperative mortality (17). In that series, the level of sacral amputation, i.e., high vs. low, did not affect the ability to achieve clear resection margins or, importantly, increase the rate of minor or major complications. The Mayo Clinic group have also reported encouraging results in nine patients undergoing high sacrectomy for recurrent rectal cancer, with R0 resection in all nine patients, 56% major morbidity and a median survival rate of 31 months.
We have previously described a technique for segmental sacrectomy involving a posterior-first disconnection of the involved sacral segments, followed by an abdominolithotomy completion exenteration (21). This is a useful technique in patients with high rectal tumours which abut only one or two sacral segments as only the involved sacral bone is resected en bloc with the tumour, avoiding a high sacrectomy and allowing preservation of uninvolved nerve roots and preservation of sacropelvic instability.
Lateral compartment
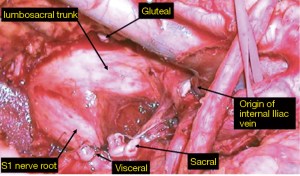
This is arguably the most difficult compartment to deal with due to the proximity of the major pelvic neurovascular structures. The presence of iliac vessels, sciatic nerve and its associated nerve roots and pelvic bone makes R0 resection difficult to achieve due to the possibility of catastrophic haemorrhage and neurological dysfunction secondary to nerve sacrifice, particularly in the setting of redo surgery and radiation damaged tissues. Despite encouraging outcomes at highly specialised centres, pelvic sidewall involvement remains a relative contraindication to surgery at many units (22-24). In 2009 a novel approach to en bloc resection of pelvic side wall structures was described (25). The side wall dissection commences at the bifurcation of the common iliac vessels at the triangle of Marcille (26). Proximal ligation of the internal iliac vessels followed by meticulous dissection and careful ligation of sidewall branches and tributaries allows the surgeon to access a more lateral plane beyond the internal iliac system (Figure 2). The dissection starts with the arterial system then the venous system, which is more lateral. Then from medial to lateral, are the nerves (lumbosacral trunk and sacral nerve roots), the muscles of the lateral compartment and finally the lateral bony pelvis which includes the ischial bone and spine. Depending on the structures involved laterally, the piriformis, internal obturator muscle, ischial spine, sciatic nerve and bony margins can then be safely resected en bloc with the aim of achieving clear margins. By routinely adopting this wider more lateral anatomical plane, which is generally not affected by previous surgery or radiotherapy, our most recent long-term data in 200 patients with lateral pelvic compartment excision has been published with a 66.5% R0 margin rate for all cancers and 68% for recurrent rectal cancer, producing a median overall survival rate of 41 months in this group of patients (27).
When the disease process involves the external or common iliac vessels, rather than shaving the tumour free of the vessel, they can be resected en bloc and reconstructed with autologous graft or synthetic graft in order to achieve a clear lateral margin (28). Chronically thrombosed external iliac vein does not necessarily require venous reconstruction as collaterals have formed prior to resection. Investigation of vascular reconstruction techniques after iliac vessel excision is ongoing at our unit and has included the novel use of saphenous vein spiral grafts (29) and bovine pericardium (Figure 3).
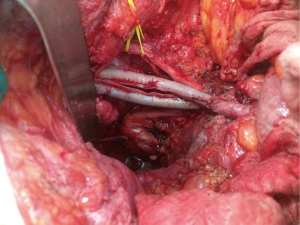
The morbidity associated with such extensive lateral resections, however, can be significant. Major morbidity has been reported in 28% of patients who undergo exenteration involving excision of the lateral pelvic compartment (27). In those who with major vascular resection (i.e., of the common or external iliac vessels), vascular-related morbidity has been reported in more than 50% of patients, with 24% requiring surgical re-intervention (28). Importantly, graft patency rates in this cohort were 96% at one year and there was no limb loss in the follow up period. If these techniques can be performed safely with a reasonable chance of R0 resection then further investigation is warranted and these patients should not be precluded from curative surgery.
Anterior compartment
There are two major considerations during exenteration involving the anterior compartment. Firstly, like in other compartments, the surgical approach must be tailored to ensure high rates of complete oncological clearance, i.e., R0 resection, and secondly, urological reconstruction remains a significant source of morbidity in the postoperative period.
Perineal urethrectomy and pubic bone excision
When operating on advanced pelvic tumours involving the anterior compartment of the pelvis, transection of the urethra in the traditional fashion from the abdominal approach in the retropubic space may risk an involved anterior margin. In our experience this is particularly problematic in male patients with recurrent rectal cancer after previous abdominoperineal resection, where the primary tumour has been dissected close to the prostate. To address this issue, ligation and division of the membranous urethra at the base of the penis from the perineal approach has been described and allows the surgeon to obtain a wider anterior surgical margin (30). The perineal approach to urethrectomy is particularly important for tumours infiltrating or abutting the pubic bone, where en bloc pubic bone resection (partial or complete) is required. This technique allows the perineal surgeon to release the obturator internus and levator ani muscles at their attachments and exposure the entire pubic symphysis and inferior pubic rami all the way laterally to the ischial tuberosities. At the same time, the abdominal surgeon exposes the superior pubic rami by releasing the anterior abdominal wall muscles, and complete or partial pubic bone excision can be performed using an oscillating saw.
Pubic bone resection was developed due to our data showing anterior recurrences as a risk factor for positive margins (17). The feasibility of radical pubic bone excision in the setting of PE has been demonstrated in a series of 29 patients (62% partial, 38% complete pubic bone excision) where R0 resection was achieved in 76% of patients with an overall survival of 53% (31). These oncological results are comparable, or even superior to, those achieved in the lateral or posterior compartments. While, similar to composite sacrectomy and lateral compartment resection, radical pubic bone resection during exenteration may associated with significant morbidity, it now represents a potential option for cure in appropriately selected patients at specialist units.
Urological reconstruction
Urinary reconstruction following PE may include proximal ureteric transection and re-implantation with or without a Boari flap following partial cystectomy, or urinary diversion in the form of a colonic or ileal conduit after radical cystectomy. Postoperative complications associated with urinary reconstruction remain a major problem in PE patients and there is limited literature on the outcomes of various reconstruction techniques, particularly regarding long-term complications like ureteric strictures. It has been demonstrated that urinary diversion following PE results in higher rates of urological complications when compared with patients who undergo cystectomy alone for primary bladder cancer (59% vs. 33%, P<0.001) (32). PE patients with primary tumours have been shown to have lower urological morbidity than those with recurrence (48% vs 67%, P=0.035) (32).
The most common urological complications following PE are urinary tract infection (36–40%) and urine leakage (11–16%), either from the uretero-enteric anastomosis or from the conduit itself (32-34). Factors that have been identified as associated with higher urological morbidity include previous radiotherapy, more extensive resections and major intraoperative blood loss (32). Although low grade urosepsis is generally managed conservatively and would not typically be considered a major surgical complication, it remains a common factor in prolonging length of hospital stay after PE at our unit. The morbidity and increased length of stay associated with urine leaks in this patient cohort have been previously reported, and may result in a shorter survival (35). Our unit developed a clinical algorithm for the diagnosis and management of urine leaks after PE in an attempt to detect leaks earlier given they are often clinically indolent. If the leak is early, within the first week, then reoperation is recommended. If the leak is delayed, then urinary diversion with percutaneous nephrostomies is performed, which increases hospital stay by 4–6 weeks (36).
Salvage surgery for re-recurrent rectal cancer
Following exenterative surgery for locally recurrent rectal cancer, a small number of patients (14% is a recent large, multicentre study) will develop isolated pelvic re-recurrence (6). The possibility of redo exenteration may represent a potentially curative option in this group of patients. Two specialist units have demonstrated the safety and oncological feasibility of re-resection in patients with second time recurrence of rectal cancer. Harji and colleagues reported a 33% R0 resection rate in 30 patients who underwent surgery for re-recurrent rectal cancer, which translated to median survival of 32 months (37,38). Colibaseanu and coworkers retrospectively reviewed 47 patients with re-recurrent rectal cancer, of which 60% had clear resection margins, with a 5-year overall survival of 33% (38). While both series reported significant morbidity, these rates are in keeping with initial exenteration outcomes and, importantly, there was no 30-day mortality in either series. These encouraging morbidity and survival outcomes are comparable with those reported for first-time exenteration and based on these data patients with re-recurrent disease should be referred to specialist centers for consideration of salvage surgery while further investigation is ongoing.
Reconstruction
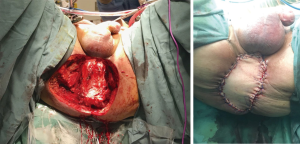
Pelvic sepsis and complications related to the perineal wound occur in approximately 10% exenteration patients, accounting for almost 40% of all postoperative complications (39). The empty space that remains following complete soft tissue exenteration promotes collection of fluid and adherence of small bowel to the denuded pelvis, which is thought to predispose patients to abscess formation, discharge from the perineal wound infection and dehiscence. Attempts to address this issue by suspending small bowel out of the pelvis with omentum, or by filling the space with myocutaneous flaps or even mammary implants, have largely failed. This so-called ‘empty pelvis syndrome’ seems to be particularly problematic where en bloc major bony resection has been performed (i.e., high sacrectomy or complete pubic bone excision), where the cut edge of bone is exposed. A previous large retrospective series comparing primary closure to myocutaneous flap repair of the perineal defect after total PE demonstrated higher rates of dehiscence and infection in the myocutaneous flap group, and furthermore in our experience a vertical rectus abdominus myocutaneous (VRAM) flap is not sufficient to fill the empty pelvis after such extended radical resections in order to preclude translocation of small bowel or perineal herniation (40). For this reason, VRAM flaps are used selective at our unit for patients with extensive skin involvement [e.g., large anal squamous cell carcinomas (SCCs), Figure 4], high sacrectomy or patients who have previous had an abdominoperineal resection for their primary cancer.
Most recently we have used a degradable synthetic mesh (GORE® BIO-A®) to reconstruct the pelvic floor. The mesh is moulded to the bony pelvic inlet from the sacral promontory to the pubic symphysis, covered with omentum superiorly and a drain placed inferiorly to the mesh (Figure 5). This effectively excludes small bowel from the exposed bony pelvis and reduces space for fluid accumulation. In our unpublished experience with 10 patients (41), two patients developed presacral collections, however, importantly, no patients had a perineal hernia, entero-perineal fistula or mesh infection requiring removal. This is the subject of ongoing investigation at our unit.
Conclusions
Due to the evolution of radical surgical techniques for PE since 1948, patients with locally advanced or recurrent pelvic tumours involving the lateral pelvic sidewall, iliac vasculature, anterior pubic bone and high sacral bone who would otherwise have be palliated are now offered a chance at cure at specialised centres. Several authors and collaborations have attempted to define the list of indications and contraindications for PE largely based on traditional anatomical and technical limitations (23,24,42,43). The indication for PE in 2018 is the reasonable chance of complete oncological resection with acceptable morbidity in the appropriate patient. This ultimately depends on patient factors, tumour biology and institutional or surgeon factors which include their personal experience and the availability of multi-disciplinary resources. The presence of metastatic disease is no longer an absolute contraindication but rather a relative contraindication, where PE can be performed in highly selected cases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Brown KGM, Solomon MJ, Koh CE. Pelvic Exenteration Surgery: The Evolution of Radical Surgical Techniques for Advanced and Recurrent Pelvic Malignancy. Dis Colon Rectum 2017;60:745-54. [Crossref] [PubMed]
- You YN, Skibber JM, Hu CY, et al. Impact of multimodal therapy in locally recurrent rectal cancer. Br J Surg 2016;103:753-62. [Crossref] [PubMed]
- Steffens D, Solomon MJ, Young JM, et al. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open 2018;2:328-35. [Crossref] [PubMed]
- Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum 2013;56:519-31. [Crossref] [PubMed]
- PelvEx Collaborative. Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br J Surg 2018;105:650-7. [Crossref] [PubMed]
- Harris CA, Solomon MJ, Heriot AG, et al. The Outcomes and Patterns of Treatment Failure After Surgery for Locally Recurrent Rectal Cancer. Ann Surg 2016;264:323-9. [Crossref] [PubMed]
- Simillis C, Baird DL, Kontovounisios C, et al. A Systematic Review to Assess Resection Margin Status After Abdominoperineal Excision and Pelvic Exenteration for Rectal Cancer. Ann Surg 2017;265:291-9. [Crossref] [PubMed]
- Young JM, Badgery-Parker T, Masya LM, et al. Quality of life and other patient-reported outcomes following exenteration for pelvic malignancy. Br J Surg 2014;101:277-87. [Crossref] [PubMed]
- Harji DP, Griffiths B, McArthur DR, et al. Surgery for recurrent rectal cancer: higher and wider? Colorectal Dis 2013;15:139-45. [Crossref] [PubMed]
- Parsons L, Bell JW. An evaluation of the pelvic exenteration operation. Cancer 1950;3:205-13. [Crossref]
- Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948;1:177-83. [Crossref] [PubMed]
- Thompson JE, Howe CW. Pelvic evisceration in the male for complication carcinoma of the rectum. N Engl J Med 1950;242:83-6. [Crossref] [PubMed]
- Koh CE, Solomon MJ, Brown KG, et al. The Evolution of Pelvic Exenteration Practice at a Single Center: Lessons Learned from over 500 Cases. Dis Colon Rectum 2017;60:627-35. [Crossref] [PubMed]
- Wanebo HJ, Marcove RC. Abdominal sacral resection of locally recurrent rectal cancer. Ann Surg 1981;194:458-71. [Crossref] [PubMed]
- Takagi H, Morimoto T, Kato T, et al. Pelvic exenteration combined with sacral resection for recurrent rectal cancer. J Surg Oncol 1983;24:161-6. [Crossref] [PubMed]
- Solomon MJ, Tan KK, Bromilow RG, et al. Sacrectomy via the abdominal approach during pelvic exenteration. Dis Colon Rectum 2014;57:272-7. [Crossref] [PubMed]
- Milne T, Solomon MJ, Lee P, et al. Assessing the impact of a sacral resection on morbidity and survival after extended radical surgery for locally recurrent rectal cancer. Ann Surg 2013;258:1007-13. [Crossref] [PubMed]
- Sasikumar A, Bhan C, Jenkins JT, et al. Systematic Review of Pelvic Exenteration With En Bloc Sacrectomy for Recurrent Rectal Adenocarcinoma: R0 Resection Predicts Disease-free Survival. Dis Colon Rectum 2017;60:346-52. [Crossref] [PubMed]
- Zacherl J, Schiessel R, Windhager R, et al. Abdominosacral resection of recurrent rectal cancer in the sacrum. Dis Colon Rectum 1999;42:1035-9; discussion 1039-40. [Crossref] [PubMed]
- Dozois EJ, Privitera A, Holubar SD, et al. High sacrectomy for locally recurrent rectal cancer: Can long-term survival be achieved? J Surg Oncol 2011;103:105-9. [Crossref] [PubMed]
- Brown KG, Solomon MJ, Austin KK, et al. Posterior high sacral segmental disconnection prior to anterior en bloc exenteration for recurrent rectal cancer. Tech Coloproctol 2016;20:401-4. [Crossref] [PubMed]
- Rahbari NN, Ulrich AB, Bruckner T, et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: is there still a chance for cure? Ann Surg 2011;253:522-33. [Crossref] [PubMed]
- Pawlik TM, Skibber JM, Rodriguez-Bigas MA. Pelvic exenteration for advanced pelvic malignancies. Ann Surg Oncol 2006;13:612-23. [Crossref] [PubMed]
- Mirnezami AH, Sagar PM, Kavanagh D, et al. Clinical algorithms for the surgical management of locally recurrent rectal cancer. Dis Colon Rectum 2010;53:1248-57. [Crossref] [PubMed]
- Austin KK, Solomon MJ. Pelvic exenteration with en bloc iliac vessel resection for lateral pelvic wall involvement. Dis Colon Rectum 2009;52:1223-33. [Crossref] [PubMed]
- Lee P, Francis KE, Solomon MJ, et al. Triangle of Marcille: the anatomical gateway to lateral pelvic exenteration. ANZ J Surg 2017;87:582-6. [Crossref] [PubMed]
- Solomon MJ, Brown KG, Koh CE, et al. Lateral pelvic compartment excision during pelvic exenteration. Br J Surg 2015;102:1710-7. [Crossref] [PubMed]
- Brown KG, Koh CE, Solomon MJ, et al. Outcomes After En Bloc Iliac Vessel Excision and Reconstruction During Pelvic Exenteration. Dis Colon Rectum 2015;58:850-6. [Crossref] [PubMed]
- Brown KG, Koh CE, Solomon MJ, et al. Spiral saphenous vein graft for major pelvic vessel reconstruction during exenteration surgery. Ann Vasc Surg 2015;29:1323-6. [Crossref] [PubMed]
- Solomon MJ, Austin KK, Masya L, et al. Pubic Bone Excision and Perineal Urethrectomy for Radical Anterior Compartment Excision During Pelvic Exenteration. Dis Colon Rectum 2015;58:1114-9. [Crossref] [PubMed]
- Austin KK, Herd AJ, Solomon MJ, et al. Outcomes of Pelvic Exenteration with en Bloc Partial or Complete Pubic Bone Excision for Locally Advanced Primary or Recurrent Pelvic Cancer. Dis Colon Rectum 2016;59:831-5. [Crossref] [PubMed]
- Brown KG, Solomon MJ, Latif ER, et al. Urological complications after cystectomy as part of pelvic exenteration are higher than that after cystectomy for primary bladder malignancy. J Surg Oncol 2017;115:307-11. [Crossref] [PubMed]
- Goldberg GL, Sukumvanich P, Einstein MH, et al. Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003). Gynecol Oncol 2006;101:261-8. [Crossref] [PubMed]
- Harada K, Sakai I, Muramaki M, et al. Reconstruction of urinary tract combined with surgical management of locally advanced non-urological cancer involving the genitourinary organs. Urol Int 2006;76:82-6. [Crossref] [PubMed]
- Teixeira SC, Ferenschild FT, Solomon MJ, et al. Urological leaks after pelvic exenterations comparing formation of colonic and ileal conduits. Eur J Surg Oncol 2012;38:361-6. [Crossref] [PubMed]
- Brown KG, Koh CE, Vasilaras A, et al. Clinical algorithms for the diagnosis and management of urological leaks following pelvic exenteration. Eur J Surg Oncol 2014;40:775-81. [Crossref] [PubMed]
- Harji DP, Sagar PM, Boyle K, et al. Outcome of surgical resection of second-time locally recurrent rectal cancer. Br J Surg 2013;100:403-9. [Crossref] [PubMed]
- Colibaseanu DT, Mathis KL, Abdelsattar ZM, et al. Is curative resection and long-term survival possible for locally re-recurrent colorectal cancer in the pelvis? Dis Colon Rectum 2013;56:14-9. [Crossref] [PubMed]
- Heriot AG, Byrne CM, Lee P, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum 2008;51:284-91. [Crossref] [PubMed]
- Jacombs AS, Rome P, Harrison JD, et al. Assessment of the selection process for myocutaneous flap repair and surgical complications in pelvic exenteration surgery. Br J Surg 2013;100:561-7. [Crossref] [PubMed]
- Lee P, Tan WJ, Brown KGM, et al. Addressing the empty pelvic syndrome following total pelvic exenteration: does mesh reconstruction help?. Colorectal Dis 2019;21:365-9. [PubMed]
- Moore HG, Shoup M, Riedel E, et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum 2004;47:1599-606. [Crossref] [PubMed]
- Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013;100:E1-33. [Crossref] [PubMed]


