Technical aspects of radiation therapy for anal cancer
Background
Anal cancer is a relatively rare tumor, with estimated new cases in 2014 in both genders likely to reach only about 7,210 (1). However, this follows the continually predicted increase over the course of recent years, and falls in line with the increasing incidence found in the past 30 years as well (2-4). The most common histologic subtype, squamous cell carcinoma (SCC), is found in up to 80-85% of cases, and has been reported to be associated with human papillomavirus (HPV)-16 in up to 93% of tumors (5-7).
Various treatment methods for anal SCC have been examined, including surgery, radiation therapy (RT), and chemotherapy with RT (CRT). Abdominoperineal resection (APR) of the rectum was the mainstay of treatment until the mid-1970’s, with 5-year survival rates that ranged between 50-70%, and local failure (LF) rates of about 50% (7,8).
Beginning in the early 1970’s, Dr. Norman Nigro developed a CRT protocol; patients were treated with RT to 30 Gray (Gy) at the primary tumor site and to the pelvic and inguinal nodes over 3 weeks, along with mitomycin C (MMC), 15 mg/m2 on day 1, and a daily dose of 1,000 mg/m2 of 5-fluorouracil (5-FU) as a continuous infusion over 4 days, the latter of which was repeated on days 29 and 32 (9). The Nigro CRT protocol demonstrated equivalent efficacy to APR, but was not compared head to head, and lead to a major shift in the management of anal SCC (7,9).
In subsequent years, multiple randomized trials were conducted to further evaluate RT for anal cancer. The United Kingdom Coordinating Committee on Cancer Research (UKCCCR) randomized 585 patients to receive either RT only at 45 Gy in 20-25 fractions (fx), or CRT, using the same RT dose with the addition of 5-FU (1,000 mg/m2 for 4 days or 750 mg/m2 for 5 days, repeated during the first and last weeks of RT) by continuous infusion; a bolus dose of MMC (12 mg/m2) was also given on day 1 to the CRT group. Those randomized to CRT had a 46% reduction in the risk of LF at 3 years compared to those who received RT alone, although with rates of 65% and 58%, respectively, there was no difference in overall survival (OS) between the groups (10).
Similarly, the European Organization for Research and Treatment of Cancer (EORTC) conducted a phase III trial that also randomized patients to either RT alone or CRT. All patients received 45 Gy in 25 fx, followed 6 weeks later by a 15 Gy boost for those with a complete response (CR), or a 20 Gy boost for partial responders. Compared to the complete remission rates of 54% for RT alone, patients who were treated with CRT had significantly higher remission rates of 80%. At 5 years, the CRT group continued to demonstrate a significantly lower rate of locoregional recurrence (LRR) (18% lower) as well as a greater colostomy-free rate (CFS) (32% higher) compared to the RT-only group (11).
In an effort to examine other chemotherapy agents to further improve the outcomes and decrease the toxicity seen in patients treated with MMC, RTOG 98-11 randomized patients to receive either the standard CRT with 5-FU and MMC, or cisplatin (CDDP), given as induction therapy with 5-FU, followed by concurrent administration with RT (12). However, the results demonstrated the 5-FU/MMC group had significantly better disease-free survival (DFS) and OS than those who received RT with 5-FU/CDDP (DFS: 67.8% versus 57.8%, OS: 78.3% versus 70.7%, respectively) (12,13).
While RTOG 98-11 examined giving CDDP and 5-FU as an induction treatment, the anal cancer trial II (ACT II) in the United Kingdom was designed to examine whether concurrent CDDP, 5-FU and RT would improve clinical outcomes and lower toxicities, and if progression-free survival (PFS) could be increased by giving an additional two courses of post-CRT maintenance 5-FU/CDDP (12,14). Through random assignment, patients received either 5-FU/MMC or 5-FU/CDDP, with or without maintenance chemotherapy of 5-FU and CDDP on weeks 11 and 14; all four groups received RT (50.4 Gy, 1.8 Gy/fx). Of the two groups who received CDDP—whether it concurrent with RT or post-CRT as maintenance therapy—neither demonstrated any benefit in PFS or OS, while toxicities remained similar, which suggested that CRT with 5-FU/MMC remain as the standard of care (14).
The standard of care should remain CRT with 50.4-59.4 Gy and 5-FU and MMC. With promising early results from multiple studies, intensity-modulated radiation therapy (IMRT) may be clinically appropriate. While local excision with wide margins can be used for early stage (T1N0M0) anal canal tumors, the 5-year cure rates are only about 60%, whereas 5-year survival rates for RT alone in early stages could be as high as 90-100%. APR, the former standard surgical management of anal cancer, is now recommended exclusively for salvage cases (15).
Anal anatomy
Approximately 3-4 cm in length, the anal canal is defined as the portion of the gastrointestinal (GI) tract which lies distal to the pelvic floor, or pelvic diaphragm (16). Along with the puborectalis muscle, anal continence is maintained via internal and external anal sphincters (16). As involuntary smooth muscle, the internal sphincter is innervated by parasympathetic pelvic splanchnic nerves. The external anal sphincter consists of skeletal muscle and surrounds the distal two thirds of the anal canal, is innervated by somatic sacral nerves, and incorporates sensation with voluntary motor control in maintenance of fecal continence (16).
The superior and inferior divisions of the anus are divided by the pectinate, or dentate, line, and are embryologically distinct, with the superior half originating from endoderm and the inferior from ectoderm (16). Hence, the anal mucosa generally consists of columnar epithelium proximal to the pectinate line and stratified squamous mucosa distally (17). A 6-12 mm mucosal transitional zone is located approximately 1 cm above the pectinate line. Here, mucosal epithelium is characterized by a combination of columnar, transitional and squamous epithelium (18). Due to distinct embryologic origins, arterial supply, innervation, and venous and lymphatic drainage also differ above and below the pectinate line.
The division between anal canal and perianal skin is marked by an abrupt shift in epithelial histology. At the anal verge, non-keratinized squamous epithelium of the anal canal transitions to keratinized, stratified squamous epithelium characteristic of skin. Malignancies arising distal to the anal verge are considered anal margin tumors, while ones proximal to the verge are defined as anal canal tumors (18).
Patterns of spread
Malignancies of the anal canal spread locally via direct extension and distantly via lymphatic or hematogenous circulation. Locoregional invasion of SCC (85-90% of anal cancers) occurs early in the natural history of anal cancer. About 10-15% of anal tumors are confined to the anal mucosa or submucosa at diagnosis (12%), while the rest presented with locally invasive disease. Approximately, one third of tumors are confined to the sphincter muscles without regional lymph node (LN) involvement (19). Half of anal cancers extend into the rectum, perianal skin, or both. Often, tumors in women invade the vaginal septum and mucosa but anovaginal fistulas are rare (<5%); while in men, the prostate gland and pelvic wall may be involved (20).
Local lymphatic spread to pelvic and inguinal nodes also occurs early in the course of disease. Three major routes of lymphatic drainage exist in the anal canal: (I) the distal rectum and most proximal part of the anal canal drain through the inferior mesenteric lymphatics toward perirectal nodes and superior hemorrhoidal nodes; (II) the proximal half of the canal (superior to the pectinate line) drains via internal iliac lymphatics to pudendal, hypogastric, obturator, inferior hemorrhoidal and middle hemorrhoidal nodes; (III) the distal portion of the canal, anal verge and perianal skin drains through external iliac lymphatics to inguinal and femoral nodes (21,22). Pelvic LN metastases have been found in approximately 30% of patients treated by APR (19,21,23). Understanding the lymphatic chains is central to RT field design.
Inguinal metastases may be detected in 15-20% of patients at initial diagnosis. Additionally they are present subclinically in 10-25% of patients, although numbers vary by institution and region (21,22). The risk of inguinal metastasis increases with primary tumor stage and proximity to the anal margin. When the primary cancer is lateralized, inguinal metastases are located ipsilaterally (24).
Extrapelvic metastases arise most frequently late in the natural course of disease and occur in the liver and lungs. At presentation, fewer than 10% of patients exhibit distant metastases (21). Local control (LC) is the primary therapeutic focus in anal cancer management due to the importance of controlling local symptoms of disease.
Patterns of failure
After definitive CRT, relapse is not uncommon. Current LRR rates are about 15-20%, while distant failure is slightly less frequent at 6-12% (25-27). Among LRR, specific rates of local and regional failures may vary somewhat. In a 25-year follow-up series of 285 cases, Tomaszewski et al. reported 15% locoregional and 6% distant relapse rates with median dose to the primary cancer of 54 Gy (25). In 43 cases of locoregional failures, 27 patients recurred at the primary site, 9 at pelvic nodes, and 12 at inguinal nodes. Eight (66.7%) of the inguinal node failures were in node-negative patients who did not receive elective (inguinal) nodal irradiation (ENI), while there were no inguinal failures in patients who underwent ENI (all to 36 Gy). Because the inguinal failure rate in patients without ENI was 1.9% in T1N0 disease versus 12.5% in T2N0 disease, ENI was recommended for tumors T2N0 or greater (25).
Das et al. retrospectively studied recurrence patterns in 167 cases of anal cancer treated definitively with CRT to a median dose of 55 Gy to the primary tumor and involved LNs while ENI was delivered to 30.6 Gy (26). LF rates were 75%, (in anus and rectum) and regional failure rates were 25% (at other sites in the pelvis and pelvic nodes). Of the regional failures, 21% were presacral and/or iliac failures, and 4% were inguinal; conversely, only 12% of patients recurred with distant metastases (26). All presacral and iliac failures occurred in patients with the superior border of the RT field at the bottom of the sacroiliac joint; therefore the authors recommended that all patients receive radiotherapy with superior field border at L5/S1. Additionally, ENI is necessary to curtail inguinal metastasis, as other studies have shown an 8-15% risk of inguinal recurrence in patients treated without ENI (24,26,28).
A similar analysis of 180 cases by Wright et al., reported a 78% LF rate, but a 44% regional failure rate, which is higher than that of the Das study (26,27). These patients were also treated for anal cancer definitively with conventional CRT, but they received a median dose of 45 Gy to the primary site and 45-50.4 Gy to involved LN depending on disease stage. This cohort experienced 30% presacral and/or iliac failures and 40% inguinal failures (26,27). All patients with inguinal recurrences received the lower dose of 45 Gy to the inguinal nodes. A proposed explanation for the high rate of inguinal relapse was the use of a lower dose of radiation to the primary and inguinal regions. However, half of the patients in Wright’s group were node negative on initial 45 Gy treatment, while Das’ node-negative patients received a lower dose of 30.6 Gy (26,27). Also, common iliac metastases comprised 20% of regional failures in the Wright study, with 100% of cases having T3 disease and 75% having node-positive disease, so this study proposes common iliac node coverage for patients with cT3-cT4 or node-positive anal cancer (27).
Positron emission tomography (PET) for planning
There have been many studies that have investigated the use of 18F-fluoro-deoxy-2-glucose positron emission tomography (18F-FDG PET) to assist in staging patients based on the presence of macroscopic disease (29). PET scan may change staging in up to 40% of cases (29,30) and is used in conjunction with a computed tomography (CT) scan of the chest, abdomen, and pelvis with IV and oral contrast (31). While 18F-FDG PET is recommended as a component of clinical staging according to the current National Comprehensive Cancer Network (NCCN) guidelines, it does not replace the need for a staging CT scan (8,31). Less clearly established though, is the use of 18F-FDG PET in treatment planning; whether indirectly, by altering the staging and subsequent RT planning and treatment, or directly, by utilizing 18F-FDG PET information to identify biologically active areas to establish potentially high-risk target volumes (29).
While the detection of positive regional LN is integral to the staging and treatment of anal cancer, the sensitivity of CT scans is only approximately 50%, while PET scans can detect up to 89% (32). PET scans detect up to 98% of primary tumors, whereas CT scans detect only 58%, although PET has not been found to significantly change T-staging for local disease which is based primarily on clinical exam of the anal tumor (30,32-34).
Additional studies have similarly found that the nodal staging adjustments resulting from PET/CT scans lead to changes in the radiation treatment plan in anywhere from 13-19% of cases (30,32). In one study, all patients with T3-4 tumors who had nodal stage changes as a result of PET/CT findings had subsequent changes in their clinical management (32). Integration of PET/CT has been utilized in treatment planning by detecting the presence or absence of high risk tissue or LN adjacent to the tumor, and thereby altering the delineation of the gross tumor volume (GTV) (29).
For instance, in one study of 39 patients (seven with anal cancer), the mean change observed to the GTV was 34% while the mean increase to the planning target volume (PTV) was 18%. Although PET/CT results did influence a change in the size of the boost volume, it did not affect the initial standard RT fields (29). PET sensitivity falls off significantly for LN less than 8 mm, which is still lower than the limits for CT or MRI; therefore, PET scans are useful for their high positive predictive value to detect disease in LN that may not be enlarged on CT (32,35).
The value of PET/CT was also examined in comparison to sentinel node biopsy (SNB), which in recent years, was found to be a valuable method of staging and planning inguinal node RT for T1-2 anal cancer (36). While PET/CT had a high sensitivity and negative predictive value of 100%, it was also found to have high false positive rates and consequently low specificity and positive predictive value (83% and 43%, respectively) compared to SNB, which was accordingly found to be better for staging nodal metastases (36). PET/CT therefore appears to be a reliable method to help localize additional foci of disease, and may help to provide detail about the extent of the primary tumor.
Simulation
Simulation of an anal cancer patient must be approached with attention to detail and knowledge of the patient’s disease prior to arrival at the simulator. Review of the patient’s findings on digital rectal examination with gynecological exam for women, inguinal nodal examination, lower endoscopic findings, and radiographic results lead the radiation oncologist to decide on the optimal positioning for each case. Common considerations are bowel sparing techniques, decision making regarding the need for bolus, genitalia sparing, and patient comfort. The administration of intravenous and oral contrast is practical for the delineation of bowel and LN regions during CT simulation.
Prone positioning with a belly board during simulation and treatment offers the advantage of improved bowel sparing but may present greater interfraction variability (37). Bladder distention prior to simulation and treatment has been shown to be quite effective in sparing bowel to an even greater extent than the belly board device, but they have an additive bowel sparing effect when used together (38). The location of the belly board aperture should be placed anteriorly to the lumbosacral spine for maximal bowel avoidance (39). On the other hand, supine positioning may be more reproducible for treatment of gross inguinal lymphadenopathy, but has greater potential for bowel in the radiation fields. In general, prone positioning is favored and should be confirmed daily with on-board imaging. Both prone and supine positioning should be conducted in the arms up position. Use of a vaginal dilator may help to reduce dose to the vagina, thereby improving side effects, though it is better tolerated when patients are positioned in a supine fashion (40). An anal marker or wires around the gross tumor or palpable LNs may be placed prior to simulation.
The “frog legged” supine position is commonly employed as it permits separation of the medial thighs to avoid unnecessary radiation dermatitis, but still allows auto-bolus of the anal region. Custom immobilization devices such as vac-loc cradles may be used to aid in reproducibility, as well as a custom foot mold depending on the length of the cradle. Involved superficial inguinal LNs may be managed with higher prescription doses and may require bolus placement. Depending on the location of the primary anal tumor, bolus may be needed to achieve the prescribed doses of radiation. For instance, a tumor that protrudes through the anal canal distally toward the perianal skin may require bolus placement. In vivo dosimetric verification using thermoluminescent dosimeters or diodes should be used to assure that dose to superficial disease is within 20% of the prescribed dose.
Delineating nodal volumes
A LN that is >1 cm in short axis diameter by CT or MRI is considered to contain metastatic disease, but the sensitivity of these imaging techniques is about 40-50% (41). Therefore, in many situations, pelvic LNs may not be enlarged but may contain microscopic tumor deposits and will require radiation treatment via the clinical target volume (CTV). A thorough understanding of the location of these LN basins in relation to the normal anatomy will aid in an accurate delineation of the CTV (42). LNs, regardless of size, generally are located adjacent to major blood vessels, which can be identified by routine CT or MRI. For this reason, vascular structures may be used as a surrogate for locating LNs (42).
Taylor et al. provide two key publications, which identify pelvic LN maps to aid the radiation oncologist in delineating LN CTVs (42,43). Recommendations are based on the visualization of ultrasmall particles of iron oxide (USPIO) given as the contrast agent for pelvic MRI for patients with 20 gynecologic tumors. Taylor recommends 7-mm margins expansions around the pelvic blood vessels. All visible nodes should be included while excluding muscle and bone from the CTV. The common iliac CTV should extend to the lateral and posterior borders of the psoas and vertebral body. The external iliac anterior border should be expanded by 10 mm along the iliopsoas muscle to include the lateral external iliac nodes, and the internal iliac LN CTV should extend laterally to the pelvic sidewall. The obturator LNs are covered with an 18-mm strip along the pelvic sidewall, and the presacral LNs require a 10-mm strip over the anterior sacrum.
Two panels, one from the Radiation Therapy Oncology Group (RTOG) and another from the Australasian Gastrointestinal Trials Group (AGITG) convened to create contouring atlases and planning guidelines for IMRT. The RTOG recommended that the primary tumor should have a 2 cm margin superiorly and inferiorly to create the CTV and coverage should include the presacrum. Regarding elective LN coverage, both the internal and external iliac LNs should be included with a 7-8 mm margin, but a margin of 10 mm ore more anterolaterally could be included if small vessels or nodes are identified. Regarding contouring normal organs, bowel loops should be tightly contoured and only delineated about 1 cm above the PTV. If there is small bowel lying within a CTV, the CTV is not modified and the portion of small bowel that fell within the target volume is not extracted from the DVHs (44).
In contrast, the AGITG provided instruction on contouring and planning for gross disease. The anatomical definitions for mesorectum and presacrum are provided. Similar to the RTOG atlas, normal LN explanations are detailed. For involved LNs, 10-20-mm margins are recommended. Recommended doses to gross disease were 54 Gy in 30 fx with simultaneous integrated boost techniques, elective volumes may be treated to 45 Gy. For a dose of 50.4 Gy for T2N0 disease, a dose of 42 Gy to elective LN volumes is reasonable (45).
The need for clarity and consistency in defining at risk nodal and organ volumes for anal cancer has driven the creation of the aforementioned contouring atlases and guidelines. Likewise, Lengelé and Scalliet detail lymphatic vessels and nodal stations relevant to the abdominal and pelvic anatomy (46). This useful guide to the nuanced pelvic anatomy is essential in depicting lymphovascular structures. The references highlighted provide guidelines for appropriate pelvic contouring for anal cancer cases.
CTV design
The RTOG panel established three elective CTV areas: CTVA (consists of the peri-rectal, pre-sacral, and internal iliac regions), CTVB (external iliac LN), and CTVC (inguinal LN), which are all recommended to be included for anal cancer RT. In brief, the CTVA should be defined caudal to the gross tumor or other involved areas at least a 2 cm margin, while the mesorectum should be covered up to the pelvic floor. An additional 1-2 cm margin to the bone should be added in tumors that extend through the levator musculature or mesorectum, and similarly around other adjacent organs that demonstrate areas of invasion. Extending to the mid-pelvis, the CTVA should include the rectum, mesentery, internal iliac region, and the bladder, the latter of which—due to daily bladder variability of location—should include an approximate 1 cm margin including the posterior bladder. The posterior-lateral margins should extend including the muscles of the pelvis sidewall, and the posterior aspect of the internal obturator vessels should be included as well. Lastly, for the upper pelvis, the entire length of rectum should be included, but depending on disease location, contouring should encompass the rectosigmoid junction, or 2 cm proximal to the most superior portion of macroscopic disease—whichever is most cephalad (44).
While the panel was in agreement that in the presence of T4 disease—determined by extension to gynecologic or genitourinary organs—both the CTVA and CTVB should be contoured, the consensus was less clear on determining CTV contours for tumors extending to be the anal canal, anal verge, perianal skin, or lower third of the vagina. The transition from CTVB to CTVC, although not well defined, is approximately at the caudad portion of the internal obturator vessels; accordingly, the CTVC should be extend 2 cm caudally from the saphenous-femoral junction (44).
Classic radiation techniques
The difficulty encountered with treatment planning for anal cancer is the need to adequately treat the inguinal LNs while not providing excess dose to the femoral heads to put them at risk of fracture. Therefore, standard anteroposterior or posteroanterior (AP-PA) or 4-field box approaches should not be employed. To circumvent this issue of dose to the inguinal LNs, Kalend et al. described the use of a transmission block with an AP-PA “pelvic wing field” (Figure 1A). The pelvic wing field consists of a wide AP under a transmission block with the anterior extended portion (hence, the “wing”) to cover the inguinal regions, while the posterior field is narrow to treat only the pelvic volume. The pelvic attenuator must be tapered at its lateral edges to reduce dose inhomogeneity at the groin (47).
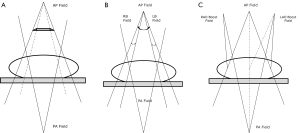
The “thunderbird” technique could be used when multileaf collimators (MLCs) with asymmetric jaws became available on the linear accelerator. In this method, the AP-PA setup is used with the patient supine. The pelvis and inguinal LNs are treated from a large AP field over the anterior pelvic region. The narrow posterior field treats only the pelvis and is matched to the skin surface, with the anterior field at the junction of the inguinals and pelvis. Two small anterior inguinal fields are used to supplement the dose to the inguinal volumes. The dose is adjusted by beam weighting (48,49).
Another technique is to employ a wide AP and narrow PA field with electron boost fields abutting the posterior exit beam. Alternatively, a standard AP-PA field could be used with electron tags. With modern day simulation, electron fields should be able to be visualized on the treatment planning system to avoid issues with missing at-risk LNs. However, there is still a risk of hot and cold spots with electron matching with photon fields.
The segmental boost techniques (SBT) and modified segmental boost techniques (MSBT) are two other methods to treat the pelvis and inguinal LN basins simultaneously. For the SBT, the isocenter is placed at the patient’s midplane (Figure 1B). A large AP field includes the pelvis and inguinal nodes. Two anterior groin boost fields are added, and a posterior pelvic field matched at the depth of the inguinal nodes, which leads to height doses above the match plane. For the MSBT, a standard large anterior field and narrow posterior field are boosted by two separate anterior oblique fields which are angled (6°-8°) to align with the divergence of the posterior field edge, which eliminates overlap from the posterior field divergence and theoretically reduces match line fibrosis (Figure 1C) (50).
In RTOG 9811, either AP-PA or multifield techniques were used to deliver a minimum total dose of 45 Gy in 25 fx, with a daily dose of 1.8 Gy. The initial radiation field was delineated by L5-S1 superiorly, and by a minimal margin of 2.5 cm around the anus and the tumor inferiorly; this field encompassed the pelvis, anus, perineum, and inguinal nodes. In order to spare the femoral heads, the lateral inguinal nodes were not routinely included in PA fields, although they were included as necessary in the lateral AP field border as determined by imaging and bony landmarks. After an initial dose of 30.6 Gy in 17 fx to the aforementioned region, the top of the field could be lowered from L5-S1 to the bottom of the sacroiliac joints if the pelvis did not contain involved LNs. Likewise, this field could then be removed from the inguinal regions after 36 Gy if inguinal LNs were negative. If, however, the pelvic LNs were involved, the pelvis would be treated to 45 Gy with a boost to positive disease to 55-59 Gy if small bowel could be excluded. Also, if the inguinal LNs were involved, then the involved inguinal side(s) would be carried to 45 Gy. For T3, T4, node positive, or T2 with residual disease after 45 Gy, a boost of 10-14 Gy could be delivered (12).
All patients received inguinal node RT; those with N0 disease received 36 Gy delivered at a minimum of 3 cm for the anterior surface, while in those with metastatic disease, the entire involved inguinal region was treated to 45 Gy, with additional boost RT. For these patients, along with any T3-4 or those with residual T2 disease after initial RT, an additional 10-14 Gy in 2-Gy fx was delivered, for a total dose of 55-59 Gy (12).
Intensity modulated radiation therapy (IMRT)
Despite the ability of the aforementioned techniques using conventional RT with 5-FU/MMC to adequately treat anal cancer, the toxicities in treating this disease with concurrent chemotherapy remain significant with effects on skin, bowel, and bone marrow (BM) when these methods are employed, as large radiation fields must be used in order to adequately cover the high risk nodal areas (51,52). Various trials by the UKCCCR, RTOG/ECOG, and EORTC demonstrated significant acute dermatologic toxicities in 48-76% of patients, GI toxicity in 33-45%, and acute hematologic toxicity (HT) (grade 3-4) in up to 61% (10-12,51,53,54). The more recent advent of IMRT, which has allowed greater variance between radiation beams, has led to a more conformal approach to targeting tumor volumes; critical normal surrounding tissues can be more effectively spared, while the radiation dose can be focused more directly to the GTV and other high-risk areas (51,55).
The proximity of the anal region relative to normal structures such as the bladder, BM, genitalia, and bowel, coupled with the fact that organ threshold RT doses are likely further reduced when receiving concurrent chemotherapy, has made it more challenging to minimize normal tissue injury (55). IMRT, however, has been shown to deliver lower doses of radiation to the surrounding normal structures than either 2D or 3D RT techniques (44), but spreads dose to normal tissues.
One of the earlier multi-center studies in which 53 patients were treated with IMRT and concurrent chemotherapy (48 of whom received the standard 5-FU/MMC combination) reported lower rates of acute skin toxicity; none had grade 4 toxicity while only 38% developed grade 3 toxicity, which was lower than the rates in patients treated with conventional CRT (48%) in RTOG 98-11. IMRT was typically set up using nine equally-spaced fields, with the aim to deliver more than 95% of the prescribed dose to the PTV. Patients received a median dose of 51.5 Gy to the primary tumor and 45 Gy to the pelvis. The dosing was remarkable since despite a greater average pelvic dose compared to that of the RTOG conventional CRT trials, the rates of GI toxicity ≥ grade 3 were only 15.1%, as opposed to the RTOG rates of 34%. Therefore, IMRT is able to spare normal bowel tissue while maintaining an elevated dose to the higher-risk areas of the pelvis, while possibly reducing toxicities and not compromising CR (92.5%), crude colostomy (10.5%), and 18-month CFS (83.7%) in this study (51). As elucidated in Table 1, IMRT appears to provide comparable LC and OS while maintaining lower rates of toxicity to normal adjacent organs (44,51).
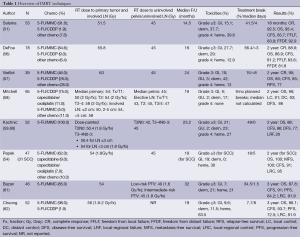
Full table
Although often attempted, comparing the specific rates of toxicities between trials can be difficult, as variances in rating scales, specific radiation or chemotherapy prescriptions, and symptomatic management can all influence the results (51,54). To help standardize the toxicity experiences between patients and trials, it has been suggested to examine the requirement for treatment breaks, which has been found in anal cancer to be associated with poorer outcomes and a predictor of disease recurrence (54,59). In a study using conventional CRT from Memorial Sloan Kettering Cancer Center, 77% of patients needed treatment breaks, and had higher rates of disease relapse (54,63). The rates of treatment breaks for chemotherapy with IMRT vary; a 2007 multi-institutional study reported that 41.5% of patients needed treatment breaks for a median length of 4 days, while a 2010 study from Duke University reported as few as 18% of their patients required breaks for a median of 5 days (51,54). Likewise, in the ACT II randomized controlled trial, the 95% CR rates were at least partially attributed to the lack of treatment breaks (14,54). The percentage of patients requiring treatment breaks with IMRT was found to be consistently lower than that with conventional CRT.
RTOG 0529 was a phase II trial designed to examine how dose-painted IMRT (DP-IMRT)—in which radiation fx size would be adjusted according to the presence of high- or low-risk disease—could reduce toxicity in patients with T2 or greater anal carcinomas. Although the primary endpoint of reducing grade ≥2 GI or genitourinary toxicities by 15% compared to the CRT group in RTOG 98-11 was not achieved, there was a significant reduction in dermatologic and GI toxicities grade ≥3 (21% versus 36%, and 23% versus 49%, respectively) and HT grade ≥2 (73% versus 85%) (12,59). A skin bolus was not necessary for DP-IMRT due to the increased skin dose secondary to the oblique beam arrangement, yet at the same time, the ability to conform the treatment around the PTV helped to reduce the skin dose over normal tissues. As a result of IMRT helping to reduce toxicity, only 49% of patients required subsequent treatment breaks; this further exhibits the importance of IMRT as a method for maintaining sphincter function and improving LC (59). Figure 2 represents a DP-IMRT case.
One issue raised in RTOG 0529 was under contouring of the CTV, which occurred over 50% of the time in the mesorectum, which has been found to be a significant area of risk for nodal spread of anal cancer; consequently, it is of upmost importance to plan the CTV accordingly (59). The dose painting technique used in RTOG 0529 was based upon tumor stage and nodal status; the tumor PTV was prescribed 50.4 Gy (1.8 Gy/fx) for T2N0 patients and 54 Gy (1.8 Gy/fx) for T3-4N0-3 disease. LN PTVs were also prescribed according to tumor stage; T2N0 patients received 42 Gy (1.5 Gy/fx) to elective LN, while T3-4N0-3 patients received 50.4 Gy (1.68 Gy/fx) or 54 Gy (1.8 Gy/fx) for nodal GTV ≤3 cm or >3 cm, respectively, and 45 Gy (1.5 Gy/fx) for elective nodal PTVs (52,59).
Dose response/toxicity
Given the ability of IMRT to adjust doses to organs at risk, investigators have studied the dosimetric correlations that may aid in predicting toxicities. The goal of meeting these dosimetric thresholds is to reduce side effects.
One known complication of anal irradiation is decrease or loss of sphincter competency. In a study of 388 prostate cancer patients included in the MRC RT01 trial, dose surface maps of the anal canal were generated, along with dose surface and dose volume histograms, and clinical endpoints were examined. Buettner et al. discovered that the strongest correlation to subjective sphincter control was the lateral extent of dose to the anal surface at 53 Gy. Cutoffs for the mean dose to the anal surface were 30-45 Gy and dose to the volume of the anal sphincter was 47 Gy (64). These relatively low doses may be challenging to achieve for anal cancer patients, as most patients will require at least a dose of 45 Gy to the anus. Similar data for prostate cancer patients treated with radiotherapy demonstrated that incontinence-related complaints show specific dose-effect relationships to individual pelvic floor muscles. Pelvic floor muscles were individually delineated with doses of ≤30 Gy to the internal anal sphincter muscle, ≤10 Gy to the external anal muscle, ≤50 Gy to the puborectalis muscle, and ≤40 Gy to the levator ani muscles to reduce the risk of bowel urgency (65).
Yi et al. have implicated the lumbosacral plexus (LSP) in pelvic toxicities also, and the incidence of toxicities is likely underrepresented. Symptoms may include new-onset burning in the low back and radiating down the leg(s). Out of 15 patients, three may have experienced symptoms possibly related to irradiation of the LSP, while the one case with lumbosacral plexopathy, was also found to have had the highest maximal dose of 58.6 Gy to the LSP (66). Bile acid malabsorption has been found after pelvic and prostate IMRT with a <1% incidence, related to irradiation of the terminal ileum (67).
Short- and long-term BM toxicity has been observed with pelvic radiotherapy, but reduced dose to the BM can lower rates of HT as detailed in Table 2.

Full table
Dosimetric parameters predictive of acute gastrointestinal toxicity in the setting of IMRT are the volume of bowel receiving 30 and 40 Gy, summarized in Table 3.
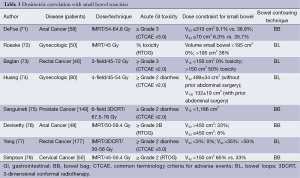
Full table
Conclusions
The treatment of anal cancer has evolved over the past 40 years, with RT, 5-FU, and MMC emerging as the optimal combination. With the evolution of newer technologies such as IMRT, therapies can be more precisely focused to deliver optimal treatment to areas of disease, while reducing toxicity and treatment breaks. The importance of sufficient contouring of nodal volumes cannot be understated; understanding LN basins, drainage patterns, and areas of high-risk disease—whether through anatomic landmarks, or PET/CT scan—can help improve the accuracy of the treatment planning volumes. New studies evaluating dose-volume parameters will contribute to the future of radiation therapy in anal cancer to further improve tolerability and permit addition of novel systemic therapies. Moving forward, additional research in the usage of PET/CT for treatment planning, and CRT combinations is necessary for continued advancement in both reducing treatment toxicity and improving disease outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. [PubMed]
- Bjørge T, Engeland A, Luostarinen T, et al. Human papillomavirus infection as a risk factor for anal and perianal skin cancer in a prospective study. Br J Cancer 2002;87:61-4. [PubMed]
- Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004;101:270-80. [PubMed]
- Blumetti J, Bastawrous AL. Epidermoid cancers of the anal canal: current treatment. Clin Colon Rectal Surg 2009;22:77-83. [PubMed]
- Glynne-Jones R, Northover JM, Cervantes A, et al. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v87-92.. [PubMed]
- Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis Colon Rectum 1984;27:763-6. [PubMed]
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. [PubMed]
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. [PubMed]
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 2013;14:516-24. [PubMed]
- Expert Panel on Radiation Oncology–Rectal/Anal Cancer, Hong TS, Pretz JL, et al. ACR Appropriateness Criteria®-Anal Cancer. Gastrointest Cancer Res 2014;7:4-14. [PubMed]
- Moore KL, Agur AM, Dalley AF. eds. Essential clinical anatomy. Philadelphia: Lippincott Williams & Wilkins, 2002.
- Sadler TW. eds. Langman’s medical embryology. Philadelphia: Lippincott Williams & Wilkins, 2011.
- Haffty BG, Wilson LD. eds. Handbook of radiation oncology: basic principles and clinical protocols. Jones & Bartlett Learning, 2009.
- Boman BM, Moertel CG, O’Connell MJ, et al. Carcinoma of the anal canal. A clinical and pathologic study of 188 cases. Cancer 1984;54:114-25. [PubMed]
- Halperin EC, Perez CA, Brady LW. eds. Perez and Brady’s Principles and Practice of Radiation Oncology. Philadelphia: Lippincott Williams & Wilkins, 2008.
- Stearns MW Jr, Urmacher C, Sternberg SS, et al. Cancer of the anal canal. Curr Probl Cancer 1980;4:1-44. [PubMed]
- Greenall MJ, Quan SH, Urmacher C, et al. Treatment of epidermoid carcinoma of the anal canal. Surg Gynecol Obstet 1985;161:509-17. [PubMed]
- Frost DB, Richards PC, Montague ED, et al. Epidermoid cancer of the anorectum. Cancer 1984;53:1285-93. [PubMed]
- Gerard JP, Chapet O, Samiei F, et al. Management of inguinal lymph node metastases in patients with carcinoma of the anal canal: experience in a series of 270 patients treated in Lyon and review of the literature. Cancer 2001;92:77-84. [PubMed]
- Tomaszewski JM, Link E, Leong T, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys 2012;83:552-8. [PubMed]
- Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys 2007;68:794-800. [PubMed]
- Wright JL, Patil SM, Temple LK, et al. Squamous cell carcinoma of the anal canal: patterns and predictors of failure and implications for intensity-modulated radiation treatment planning. Int J Radiat Oncol Biol Phys 2010;78:1064-72. [PubMed]
- Ferrigno R, Nakamura RA, Dos Santos Novaes PE, et al. Radiochemotherapy in the conservative treatment of anal canal carcinoma: retrospective analysis of results and radiation dose effectiveness. Int J Radiat Oncol Biol Phys 2005;61:1136-42. [PubMed]
- Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 2003;57:853-63. [PubMed]
- Nguyen BT, Joon DL, Khoo V, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol 2008;87:376-82. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines In Oncology: Anal Carcinoma Version 2. 2014. Available online: http://www.nccn.org/professionals/physician_gls/pdf/anal.pdf
- Ed Winton. The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer 2009;100:693-700. [PubMed]
- Cotter SE, Grigsby PW, Siegel BA, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol Biol Phys 2006;65:720-5. [PubMed]
- Trautmann TG, Zuger JH. Positron Emission Tomography for pretreatment staging and posttreatment evaluation in cancer of the anal canal. Mol Imaging Biol 2005;7:309-13. [PubMed]
- Anderson C, Koshy M, Staley C, et al. PET-CT fusion in radiation management of patients with anorectal tumors. Int J Radiat Oncol Biol Phys 2007;69:155-62. [PubMed]
- Mistrangelo M, Pelosi E, Bellò M, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of inguinal node metastases in patients with anal cancer. Int J Radiat Oncol Biol Phys 2010;77:73-8. [PubMed]
- Das IJ, Lanciano RM, Movsas B, et al. Efficacy of a belly board device with CT-simulation in reducing small bowel volume within pelvic irradiation fields. Int J Radiat Oncol Biol Phys 1997;39:67-76. [PubMed]
- Kim TH, Chie EK, Kim DY, et al. Comparison of the belly board device method and the distended bladder method for reducing irradiated small bowel volumes in preoperative radiotherapy of rectal cancer patients. Int J Radiat Oncol Biol Phys 2005;62:769-75. [PubMed]
- Lee SH, Kim TH, Kim DY, et al. The effect of belly board location in rectal cancer patients treated with preoperative radiotherapy. Clin Oncol (R Coll Radiol) 2006;18:441-6. [PubMed]
- Briere TM, Crane CH, Beddar S, et al. Reproducibility and genital sparing with a vaginal dilator used for female anal cancer patients. Radiother Oncol 2012;104:161-6. [PubMed]
- Williams AD, Cousins C, Soutter WP, et al. Detection of pelvic lymph node metastases in gynecologic malignancy: a comparison of CT, MR imaging, and positron emission tomography. AJR Am J Roentgenol 2001;177:343-8. [PubMed]
- Taylor A, Rockall AG, Reznek RH, et al. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:1604-12. [PubMed]
- Taylor A, Rockall AG, Powell ME. An atlas of the pelvic lymph node regions to aid radiotherapy target volume definition. Clin Oncol (R Coll Radiol) 2007;19:542-50. [PubMed]
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824-30. [PubMed]
- Ng M, Leong T, Chander S, et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys 2012;83:1455-62. [PubMed]
- Lengelé B, Scalliet P. Anatomical bases for the radiological delineation of lymph node areas. Part III: Pelvis and lower limbs. Radiother Oncol 2009;92:22-33. [PubMed]
- Kalend AM, Park TL, Wu A, et al. Clinical use of a wing field with transmission block for the treatment of the pelvis including the inguinal node. Int J Radiat Oncol Biol Phys 1990;19:153-8. [PubMed]
- Ma L, Chang W, Lau-Chin M, et al. Using static MLC fields to replace partial transmission cerrobend blocks in treatment planning of rectal carcinoma cases. Med Dosim 1998;23:264-6. [PubMed]
- Dittmer PH, Randall ME. A technique for inguinal node boost using photon fields defined by asymmetric collimator jaws. Radiother Oncol 2001;59:61-4. [PubMed]
- Moran M, Lund MW, Ahmad M, et al. Improved treatment of pelvis and inguinal nodes using modified segmental boost technique: dosimetric evaluation. Int J Radiat Oncol Biol Phys 2004;59:1523-30. [PubMed]
- Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 2007;25:4581-6. [PubMed]
- Kachnic LA, Tsai HK, Coen JJ, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012;82:153-8. [PubMed]
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. [PubMed]
- Pepek JM, Willett CG, Wu QJ, et al. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys 2010;78:1413-9. [PubMed]
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354-61. [PubMed]
- DeFoe SG, Beriwal S, Jones H, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal carcinoma--clinical outcomes in a large National Cancer Institute-designated integrated cancer centre network. Clin Oncol (R Coll Radiol) 2012;24:424-31. [PubMed]
- Vieillot S, Fenoglietto P, Lemanski C, et al. IMRT for locally advanced anal cancer: clinical experience of the Montpellier Cancer Center. Radiat Oncol 2012;7:45. [PubMed]
- Mitchell MP, Abboud M, Eng C, et al. Intensity-modulated Radiation Therapy With Concurrent Chemotherapy for Anal Cancer: Outcomes and Toxicity. Am J Clin Oncol 2013. [Epub ahead of print]. [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [PubMed]
- Kachnic L, Winter K, Myerson R, et al. Early Efficacy Results of RTOG 0529: A Phase II Evaluation of Dose-painted IMRT in Combination with 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Carcinoma of the Anal Canal. Int J Radiat Oncol Biol Phys 2010;78:S55.
- Bazan JG, Hara W, Hsu A, et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011;117:3342-51. [PubMed]
- Chuong MD, Freilich JM, Hoffe SE, et al. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Carcinoma of the Anal Canal. Gastrointest Cancer Res 2013;6:39-45. [PubMed]
- Roohipour R, Patil S, Goodman KA, et al. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum 2008;51:147-53. [PubMed]
- Buettner F, Gulliford SL, Webb S, et al. The dose-response of the anal sphincter region--an analysis of data from the MRC RT01 trial. Radiother Oncol 2012;103:347-52. [PubMed]
- Smeenk RJ, Hoffmann AL, Hopman WP, et al. Dose-effect relationships for individual pelvic floor muscles and anorectal complaints after prostate radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:636-44. [PubMed]
- Yi SK, Mak W, Yang CC, et al. Development of a standardized method for contouring the lumbosacral plexus: a preliminary dosimetric analysis of this organ at risk among 15 patients treated with intensity-modulated radiotherapy for lower gastrointestinal cancers and the incidence of radiation-induced lumbosacral plexopathy. Int J Radiat Oncol Biol Phys 2012;84:376-82. [PubMed]
- Harris V, Benton B, Sohaib A, et al. Bile acid malabsorption after pelvic and prostate intensity modulated radiation therapy: an uncommon but treatable condition. Int J Radiat Oncol Biol Phys 2012;84:e601-6. [PubMed]
- Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1431-7. [PubMed]
- Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83-90. [PubMed]
- Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;79:800-7. [PubMed]
- DeFoe SG, Kabolizadeh P, Heron DE, et al. Dosimetric parameters predictive of acute gastrointestinal toxicity in patients with anal carcinoma treated with concurrent chemotherapy and intensity-modulated radiation therapy. Oncology 2013;85:1-7. [PubMed]
- Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol 2003;69:201-7. [PubMed]
- Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:176-83. [PubMed]
- Huang EY, Sung CC, Ko SF, et al. The different volume effects of small-bowel toxicity during pelvic irradiation between gynecologic patients with and without abdominal surgery: a prospective study with computed tomography-based dosimetry. Int J Radiat Oncol Biol Phys 2007;69:732-9. [PubMed]
- Sanguineti G, Endres EJ, Sormani MP, et al. Dosimetric predictors of diarrhea during radiotherapy for prostate cancer. Strahlenther Onkol 2009;185:390-6. [PubMed]
- Devisetty K, Mell LK, Salama JK, et al. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother Oncol 2009;93:298-301. [PubMed]
- Yang TJ, Oh JH, Son CH, et al. Predictors of acute gastrointestinal toxicity during pelvic chemoradiotherapy in patients with rectal cancer. Gastrointest Cancer Res 2013;6:129-36. [PubMed]
- Simpson DR, Song WY, Moiseenko V, et al. Normal tissue complication probability analysis of acute gastrointestinal toxicity in cervical cancer patients undergoing intensity modulated radiation therapy and concurrent cisplatin. Int J Radiat Oncol Biol Phys 2012;83:e81-6. [PubMed]


