
Is there difference between anastomotic site and remnant stump carcinoma in gastric stump cancers?—a single institute analysis of 90 patients
Introduction
Balfour was the first to elucidate the effects of gastric reconstruction on development of gastric stump carcinoma (GSC) (1). Since then many authors especially those from China and Europe have tried to explain the pathophysiology behind development of GSC through their reviews. However, many have been published in local languages and hence difficult to interpret globally (2-8). Also extensive comparison has been done between GSC caused due to previous benign disease versus those developing after being operated for malignant disease several years back (9). Another group of authors have tried to compare GSC with proximal gastric cancers (10-12). Nonetheless a few of these papers have tried to differentiate between malignancies that develop at the anastomotic site (ASC) from those that develop at the remnant stump (RSC). Little has been reported regarding differences between these two different entities since Sinning et al. (13) in 2006 recognized the pathophysiological differences in the malignancy that developed at ASC from those of RSC.
The purpose of our study was to compare clinical, pathological and survival characteristics of ASC patients with those of RSC patients. In this study which is the only study from Indian subcontinent to the best of our knowledge, we have applied 7th TNM staging system to describe pathological, clinical and survival analysis of patients presenting with GSC with respect to anatomical site of presentation (ASC versus RSC).
Methods
After institutional review board approval (KMIO/MEC/007/25.November 2017) retrospective analysis was done for patients who underwent surgery for GSC between January 2005 and December 2017. Of the total 112 patients, 22 patients were excluded from the study due to extensive loss of data. Ninety patients underwent curative resection and were evaluated based on anatomic site at which they developed malignancy. Patients who presented with metastatic disease were excluded from the study. Eight patients who were found to have metastatic disease during surgery have also been excluded from the analysis due to grossly unavailable data. Anastomotic site and RSC carcinoma were defined based upon the location of bulk of the disease. Mere extension of the disease which occupied major part of body/antro-pylorus into the anastomotic site was not termed as anastomotic disease and vice versa.
Clinical parameters compared were age, gender, previous site of ulcer, type of previous surgery (vagotomy with Billroth II reconstruction with intact antro-pyloric region or distal gastrectomy with Billroth II reconstruction) and interval between previous surgery with development of GSC. Most of the patients while recording history could not remember the type of surgery that was performed previously and was confirmed by endoscopic and intraoperative findings on the data record sheet. No patient received Billroth I reconstruction in our series. Treatment related variables were resection status (R0/R1), type of lymphadenectomy (D1/D2) and splenectomy status during surgery. A number of pathological variable were analyzed such as total number of nodes extracted, number of nodes positive for metastasis, ratio of positive to total nodes extracted (>20 and <20), status of mesenteric nodes, Laurens classification (intestinal type, diffuse type or unspecified), H. Pylori, pT, pN and TNM staging as per 7th edition of AJCC on cancer staging system.
Statistical analysis
Survival assessment was performed using Kaplan Meier analysis. Cox Proportional Hazard Model was used for studying prognostics variables. Log rank test was used to compare the survival curves. Chi square test was used to study the association between quantitative data. Any P value <0.05 was considered as statistically significant.
Results
Clinical parameters (Table 1)

Full table
Demographic data is provided in Table 1. High incidence of malignancy developed in those who underwent vagotomy with gastrojejunostomy (V + GJ) compared to those who had distal gastrectomy + Billroth II (DG+BII) (90%, P=0.015). ASC was significantly associated with development Lauren’s diffuse type cancer (63.2%) compared to RSC (18.2%, P=0.0001). H. Pylori seemed more strongly associated with RSC (72.7%) compared to ASC (15.8%, P=0.001).
Pathology and staging (Tables 1,2)
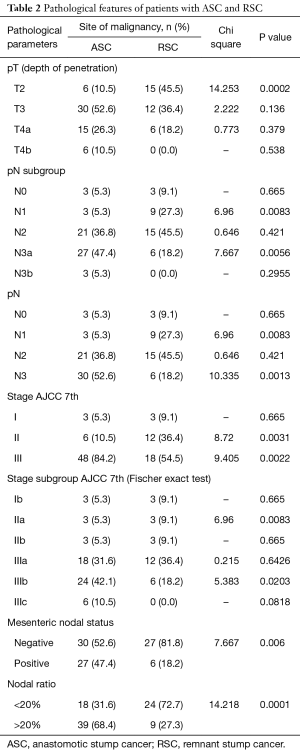
Full table
Detailed pathological assessment and staging results are provided in Tables 1,2. However notable ones are mentioned here. Sixty-nine patients (76.6%) had pT3 and above disease. Overall 80% of patients (n=72) had pN2 and above disease, however pN3 nodal metastasis was significantly higher for ASC (P=0.0013). Also nodal ratio of greater than 20 was highly associated with ASC (P=0.0001). Since patients with metastatic disease have been excluded from the study only stages I to III have been analyzed. In a subgroup analysis of stage, RSC patients significantly contributed to stage IIa (P=0.0083) whereas stage IIIb had significantly higher number of patients with ASC (P=0.0203). Close to half number of patients (n=27, 47.4%) with ASC presented with positive mesenteric nodes and was significantly higher compared to RSC (n=6, P=0.006).
Survival analysis (Table 1,Figures 1,2)
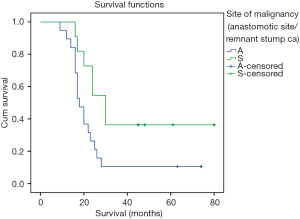

The overall survival curves for ASC and RSC are shown in Figure 1. Overall median survival was 20 months (range, 16.9–23.09 months). Patients with ASC had poorer median survival (18 months; range, 16.4–19.6 months) compared to those with RSC (30 months; range, 25.5–34.5 months, log rank =0.000). Overall 3-year survival and 5-year survival was 20% and 13.3% respectively. Three-year survival was significantly lower (10.5%) for patients with ASC compared to those with RSC (36.4%, P=0.003). Patients with pT4b, stage III, positive mesenteric node and nodal ratio of >20 were associated with worst survival (Figures 1,2).
Discussion
Before the era of proton pump inhibitors, the most commonly performed surgery for peptic ulcer in India was vagotomy and drainage procedure. However, antrectomy was also performed for patients who presented with bleeding gastric ulcers along with Billroth II reconstruction (14). Both these surgeries had at least two things in common, one that they both were performed mostly for ulcer and secondly both required gastrojejunostomy—a procedure that diverted the jejunal bile into stomach and changed its microenvironment to a great extent. Duodenogastric reflux has been cited as the major cause for GSC by a number of authors. Vagotomy leads to hypochlorydia and increased epithelial proliferation rendering the mucosa more prone to DNA damage and resultant gastric cancer (15).
Sinning et al. (13) in their succinct review on GSC have described an altogether different pathogenesis for cancers developing at two different sites- anastomotic site and remnant gastric stump. Diffuse type carcinoma develops in premalignant lesions involving adenoid cystic proliferation at the anastomotic site due to duodenogastric reflux. Intestinal type carcinoma develops at the body of gastric stump which is preceded by stump dysplasia that progressively loses its gastric phenotype (13). However, Morgagni et al. did not find any such relationship in their study (16).
Not just the pathogenesis and histology but these two differ in multiple clinical parameters including extent of nodal involvement, staging and survival as evident by our analysis. Multiple authors presented their series of GSC where they found that anastomotic site malignancy developed significantly more in patients who were previously operated for benign disease (11,17,18). Even though we failed to find any such correlation, in Cox proportional hazard model prior malignant disease was associated with poor survival (HR =1.797, P=0.049) (Table 3).
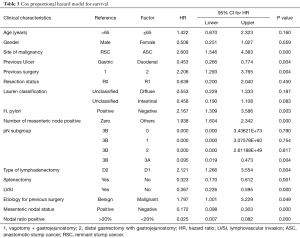
Full table
Păduraru et al. stated that H. pylori infection does not seem to be an important risk factor for development of GSC however they themselves noted that this finding may be controversial (15). We found that H. pylori was more significant in development of non-anastomotic site cancer rather than the anastomotic site. Ohira et al. demonstrated that H. pylori prevalence was less where duodenogastric reflux was the primary cause for malignancy i.e., anastomotic site which conforms with our finding (19).
Multiple authors have demonstrated that involvement of mesenteric nodes confers poor survival to those with GSC. In our study we found that patients with anastomotic site cancers had much higher prevalence of mesenteric LN metastasis and also that it contributed to poor 3-year survival (18,20). Shimada et al. in his review concluded that LN involvement of jejunal mesentery in GSC was between 7% and 46.8% (21). We found an incidence of 36.7% that is well within this range. Moreover, an important aspect we noted was that the incidence was significantly higher in anastomotic group than the non-anastomotic one. Greater than two thirds of patients presented with stage III disease especially those with anastomotic site cancer. Our findings are in line with those of Huang et al. (9).
Since more than 85% of our patients succumbed to the disease within 5 years of treatment, we calculated their 3-year survival curves (Figures 1,2). Compared to previous authors our median survival time was less (20 versus 30.9 months) (20). However, there was a significant difference between survival of anastomotic site (18 months) and body site malignancy (30 months) that has not been highlighted by most of the authors. Stage wise deterioration of survival too occurred which was worst with anastomotic site cancers (9) (Figure 2).
Conclusions
Our study shows that cancer originating at the anastomotic site is substantially different than that at the body site of GSC. ASC is more aggressive disease compared to RSC and has different pathology, higher rates of nodal involvement (both primary and mesenteric), presents with higher stage and has worst 3-year survival. An early surveillance plan for patients who have undergone Billroth II reconstruction should be in place after 10 years of initial surgery (14). We suggest that early recognition of patients with anastomotic site cancer would help to improve survival by undertaking aggressive management.
Acknowledgements
We would like to thank Dr. Durgesh Sahoo for his assistance with statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study has been approved by Institutional ethics committee for assessment of retrospective data (KMIO/ MEC/007/25.November 2017).
References
- Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg 1922;76:405-8. [Crossref] [PubMed]
- Gao Z, Li Y, Jiang K, et al. Progress and controversy on diagnosis and treatment of gastric stump cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:588-92. [PubMed]
- Li Y, Gao Z, Zhao X, et al. Meta-analysis of gastric stump cancer after gastrectomy for gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:569-77. [PubMed]
- Wang Y, Li Z, Jin C, et al. Clinicopathological characteristics and prognostic factor analysis of carcinoma in remnant stomach cancer at Peking University Cancer Hospital. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:522-8. [PubMed]
- Chen L. Epidemiological characteristics and inducing factors of gastric stump cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:498-501. [PubMed]
- Gao Z, Jiang K, Ye Y, et al. Interpretation on Chinese surgeons' consensus opinion for the definition of gastric stump cancer (version 2018). Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:486-90. [PubMed]
- Nienhüser H, Blank S, Sisic L, et al. Gastric stump carcinoma: frequency, treatment, complications and prognosis. Chirurg 2017;88:317-27. [PubMed]
- Vajda D, Nagy E, Molnar G. Gastric stump carcinoma. Fortschr Geb Rontgenstr Nuklearmed 1960;92:653-8. [Crossref] [PubMed]
- Huang H, Wang W, Chen Z, et al. Prognostic factors and survival in patients with gastric stump cancer. World J Gastroenterol 2015;21:1865-71. [Crossref] [PubMed]
- Takeno S, Hashimoto T, Maki K, et al. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol 2014;20:13734-40. [Crossref] [PubMed]
- Tokunaga M, Sano T, Ohyama S, et al. Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancer. J Gastrointest Surg 2013;17:313-8. [Crossref] [PubMed]
- Rabin I, Kapiev A, Chikman B, et al. Comparative study of the pathological characteristics of gastric stump carcinoma and carcinoma of the upper third of the stomach. Isr Med Assoc J 2011;13:534-6. [PubMed]
- Sinning C, Schaefer N, Standop J, et al. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol 2007;33:133-9. [Crossref] [PubMed]
- Balraj V, Perakath B. Post-gastric surgery: is a closer follow up required? Natl Med J India 2001;14:251-2. [PubMed]
- Păduraru DN, Nica A, Ion D, et al. Considerations on risk factors correlated to the occurrence of gastric stump cancer. J Med Life 2016;9:130-6. [PubMed]
- Morgagni P, Gardini A, Marrelli D, et al. Gastric stump carcinoma after distal subtotal gastrectomy for early gastric cancer: experience of 541 patients with long-term follow-up. Am J Surg 2015;209:1063-8. [Crossref] [PubMed]
- Irino T, Hiki N, Ohashi M, et al. Characteristics of gastric stump cancer: A single hospital retrospective analysis of 262 patients. Surgery 2016;159:1539-47. [Crossref] [PubMed]
- Di Leo A, Pedrazzani C, Bencivenga M, et al. Gastric stump cancer after distal gastrectomy for benign disease: clinicopathological features and surgical outcomes. Ann Surg Oncol 2014;21:2594-600. [Crossref] [PubMed]
- Ohira M, Toyokawa T, Sakurai K, et al. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol 2016;22:2424-33. [Crossref] [PubMed]
- Thorban S, Böttcher K, Etter M, et al. Prognostic factors in gastric stump carcinoma. Ann Surg 2000;231:188-94. [Crossref] [PubMed]
- Shimada H, Fukagawa T, Haga Y, et al. Does remnant gastric cancer really differ from primary gastric cancer? A systematic review of the literature by the Task Force of Japanese Gastric Cancer Association. Gastric Cancer 2016;19:339-49. [Crossref] [PubMed]


