Typical carcinoids, goblet cell carcinoids, mixed adenoneuroendocrine carcinomas, neuroendocrine carcinomas and adenocarcinomas of the appendix: a comparative analysis of survival profile and predictors
Introduction
Neuroendocrine cells are ubiquitous cells which are widely distributed throughout the body (1). Tumors arising from these cells—neuroendocrine neoplasms—are therefore a heterogenous group with a wide range of clinical behaviors (1-3). Due to the variation in their behavior, newer classifications are constantly emerging with the goal to clarify the features of these tumors. In the World Health Organization (WHO) classification scheme, the Ki-67 proliferative index and mitotic count are crucial prognostic factors and used in histologic classification (4). In the 2010 update, mixed adenoneuroendocrine carcinomas (MANEC) were identified as a distinct subtype of neuroendocrine tumors (NET) (4,5). Neuroendocrine carcinomas (NEC), which consist of small cell and large cell carcinomas, were also classified as distinct group of poorly differentiated tumors with high Ki-67 index greater than 20% (4).
Despite the newer classifications with advances in biological and histological characterization, treatment for the presumed more aggressive histologic subtypes is not well defined (3). Goblet cell carcinoids (GCC) and other atypical NET of the appendix are usually managed with the same algorithm as colon cancers: a treatment design that is also applied to adenocarcinomas of the appendix (6). This invariably implies that the presence of atypical pathology in NET of the appendix necessitates a more extensive approach to care. This is because these tumors are thought to have poorer outcomes and hence need more aggressive care. To date, few studies have looked at the clinicopathologic features and the survival profile of these newer histological groups of NET. In this light, this study sets out to investigate the clinical and pathologic features of both appendiceal NET and adenocarcinomas using the latest histopathologic classification. It further aims to examine the survival profile of the various NET subtypes in comparison to that of appendiceal adenocarcinomas.
Methods
Using the Surveillance, Epidemiology, and End Results (SEER) database, cases of appendiceal tumors for patients who were 18 years and above from 2010 till 2014 were reported. The data which were obtained include patients’ demographics, clinical information and pathological details of disease. Using the International Classification of Diseases for Oncology 3 (ICD-O-3), appendiceal tumors of interest were typical carcinoids (TC), GCC, NEC, MANEC and adenocarcinomas. To allow for uniformity of staging and comparison, this study was designed to review cases after the emergence of the American Joint Committee on Cancer (AJCC) 7th edition staging and the 2010 WHO new classification.
Mean and standard deviation were used to describe continuous variables while categorical variables were presented as proportions. Quantitative data were analyzed using the Fisher’s t-test and the Chi-square test was used to compare the categorical variables. Overall survival (OS) was defined as the time from diagnosis to the time of death. Survival analysis was done with Kaplan-Meier curves. Differences in survival curves were compared using log-rank test.
The impact of independent variables on survival over time was examined using the Cox proportional hazards regression model. This model was generated using apposite variables determined a priori. Other independent variables identified by univariate analyses (P<0.20), were also added to the model. Analyses were conducted using IBM SPSS Statistics v. 22 and statistical significance was set at P<0.05.
Results
Patient characteristics
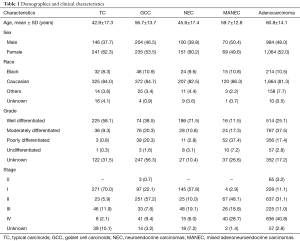
Majority of the patients who were reviewed were females, with female-to-male ratio of 1.2. Females outnumbered males in a 1.3 to 1 ratio in the NET subgroup and 1.1 to 1 ratio in the adenocarcinoma group. The mean age at diagnosis for all NET was 50.3±17 years while that of adenocarcinomas was 60.8±14.1 years. Within the NET subgroups, the mean ages for TC, GCC, NEC and MANEC were 42.9±17.3, 56.7±13.7, 45.6±17.4 and 59.7±12.8 years, respectively. Eighty-one and one third percent (81.3%) of the patients who were diagnosed with adenocarcinoma were white, 10.5% were black and 7.7% were of other races (P=0.000). There were 10 cases documented as unknown race (0.5%). The observation of white preponderance was maintained in both the NET group and adenocarcinomas. Patients’ demographics are shown in Table 1.
Full table
Tumor characteristics
Reviewing the adenocarcinomas, 25.1%, 37.5%, 17.4% and 2.8% were well differentiated, moderately differentiated, poorly differentiated and undifferentiated, respectively. Histologic grades were missing for 17.2% of the adenocarcinomas. In a subgroup analysis of the NET, most of the TC were well differentiated (58.1%) while 9.3%, 0.8% and 0.3% were moderately differentiated, poorly differentiated and undifferentiated, respectively. NEC were mostly well differentiated (71.5%) and 10.8%, 2.8% and 3.1% were moderately differentiated, poorly differentiated and undifferentiated, respectively. For MANEC, 11.5%, 17.3%, 37.4% and 7.2% were well differentiated, moderately differentiated, poorly differentiated and undifferentiated, respectively. GCC cases with documented grades were mostly well differentiated (38.5%). Most cases of GCC had an unknown histologic grade (56.3%). These clinicopathologic features are shown in Table 1.
Majority of the adenocarcinomas were diagnosed at stage IV (40.8%). Stage 0 constituted 3.2% while stages I, II and III were 11.1%, 31.1% and 11.0%. Most MANEC were diagnosed at stages II and IV (48.1% and 28.7% respectively). NEC and TC presented mostly at stage I (57.8% and 70%, respectively) while GCC was mostly diagnosed at stage II (57.2%) (Table 1).
Overall survival
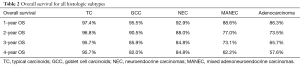
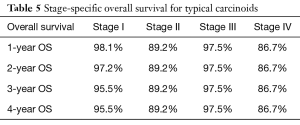
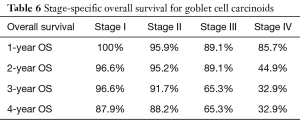
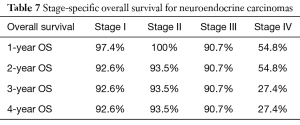
Overall survival for adenocarcinoma using the Kaplan-Meier curve was 86.3%, 73.5%, 65.7%, and 57.6% for 1-, 2-, 3- and 4-year OS, respectively. Comparing the various NET histologic subtypes, TC showed better survival profile with 1-year and 4-year overall survival of 97.4% and 95.7%, respectively while MANEC had the worst survival outcome with 1- and 4-year overall survival of 88.6% and 62.2%, respectively. GCC had a better 1-year overall survival compared to NEC (95.5% versus 92.9%) but showed slightly worse 4-year OS (82% versus 84.8%) (Table 2).
Full table
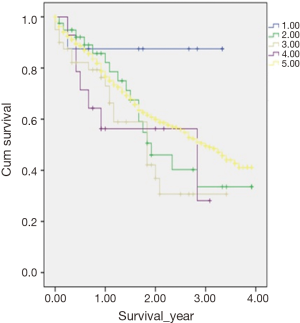
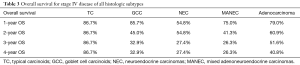
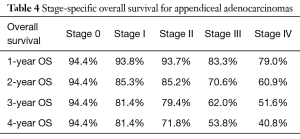
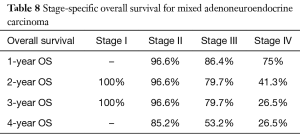
In a subanalysis, 1-year OS for stage IV of the different histologic subtypes of appendiceal NET were 86.7%, 85.7%, 54.8% and 75% for TC, GCC, NEC and MANEC, respectively. The 4-year OS for stage IV disease were 86.7%, 32.9%, 27.4% and 26.3% for TC, GCC, NEC and MANEC, respectively (Table 3). Adenocarcinomas, on the other hand, had 1-year OS of 94.4%, 93.8%, 93.7%, 83.3% and 79% for stages 0 to IV, respectively while the 4-year overall survival were 94.4%, 81.4%, 71.8%, 53.8% and 40.8% for stages 0, I, II, III and IV, respectively (Table 4). Kaplan-Meier representation of the OS for stage IV diseases is shown in Figure 1. Stage-specific overall survival for the various NET histologic subtypes are shown in Tables 5-8.
Full table
Full table
Full table
Full table
Full table
Full table
Predictors of overall survival
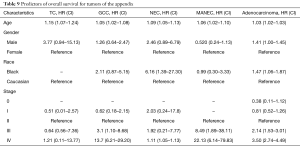
Using the Cox proportion hazards regression model, age at diagnosis (HR 1.03), African-American race (HR 1.47) and stage IV disease (HR 9.58) were independent predictors of survival for appendiceal adenocarcinoma (Table 9). For NET, advanced age at diagnosis, advanced disease stage and the African-American race were identified as negative independent predictors of survival (Table 9).
Full table
Discussion
Cancers of the appendix are rare tumors. They are usually found incidentally after appendectomies (7,8). In a review using the SEER database, they were noted to make up about 1% of GI tumors (7,8). Despite their rarity, histologic examination shows that tumors of the appendix display a wide range of features that span from purely neuroendocrine to epithelial tumors (9,10). A review of appendiceal tumors showed that TC are more common than appendiceal adenocarcinomas (7). In this study, there were more appendiceal adenocarcinomas than TC. While this finding might highlight appendiceal adenocarcinomas as more common, it is important to note that a preferential reporting of malignant tumors in the SEER database (11,12) might be responsible for this differential higher prevalence of the adenocarcinomas. Furthermore, GCC, NEC and MANEC are very rare tumors that occur less commonly than adenocarcinomas (10).
Gender distribution in appendiceal adenocarcinomas has shown varying results based on different studies in the published literature. Some studies documented higher incidences in men while others reported a higher female to male ratio (13,14). This study showed a slight preponderance of females in the adenocarcinoma group. The same pattern of distribution was also noted in the different neuroendocrine tumor histologic subtypes (P=0.001). GCC and MANEC, however, have not shown gender predisposition. Some studies reported a higher proportion of male versus female while other studies reported female preponderance (5,6,15).
Caucasians were affected more than African-Americans and other races in this study. This disparity was noted in all histologic subtypes of NET. Although race-adjusted incidence, which would have given a better reflection of the effect of race, was not calculated, race was noted to be a predictor of survival in adenocarcinoma and NEC. The African-American race had a negative impact on survival with HRs of 1.47 and 6.6 for adenocarcinoma and NEC, respectively (P=0.022 and 0.017). On a general note, the African-American race appears to be a negative predictor of OS. However, it is pertinent to note that disparities in health care access may also contribute to the observed difference.
Prior studies have suggested that atypical NET (GCC, NEC and MANEC) are more aggressive than TC and usually present at more advanced stages (6). In this study, however, we observed that most of the GCC, MANEC and NEC were diagnosed at stages I and II. Appendiceal adenocarcinoma, on the other hand, presented mostly at stage IV. With respect to overall survival, results from this study showed that atypical histologic subtypes of NETs have less favorable outcome. In fact, the overall survival for MANEC is comparable to that of adenocarcinoma. Brathwaite et al., in their study, observed no difference in overall survival between MANEC, GCC and signet cell carcinomas (6).
Although a better overall survival was noted for GCC, NEC and MANEC when compared to adenocarcinoma, this benefit was lost in stage IV disease. Our analysis showed that stage IV adenocarcinoma had a better 1-year OS compared to NEC and MANEC (79% versus 75% and 54.8%, respectively). There was also a trend toward better overall survival for stage IV adenocarcinoma compared to stage IV GCC, NEC and MANEC, with 4-year OS of 40.8%, 32.9%, 27.4% and 26.3%, respectively.
Since its description, various authors have tried to elucidate the defining characteristics of MANEC. This study confirmed findings from the limited published literature that indicated worse overall survival for MANEC compared to GCCs and TCs (6). However, there is paucity of studies comparing MANEC to NEC and adenocarcinomas. Results from our analysis showed that NEC with 84.8% 4-year OS had better survival when compared to MANEC (4-year OS 62.2%). Furthermore, the median overall survival for MANECs was 3.1 years (95% CI, 2.88–3.38), 3.57 years (95% CI, 3.42–3.72) for NEC and 3.0 years for adenocarcinomas (95% CI, 2.93–3.07).
Using the Cox proportion hazards regression model, advanced age and advanced disease stage were identified as independent negative predictors of overall survival for NET and adenocarcinoma.
This study used a population-based database which allows for generalizability of its findings and reduction in selection bias. Furthermore, by utilizing the most recent histopathological diagnosis criteria, this study examined the clinicopathological characteristics of appendiceal NETs and adenocarcinomas therefore providing a basis for comparison for future studies. Despite its merits, this is a retrospective study with associated recall or documentation bias. Furthermore, SEER database does not provide granular details regarding the use of chemotherapy and other treatments modalities that could influence the various outcomes considered in this study.
Conclusions
To the best of our knowledge, this is the most comprehensive comparative analysis of NET of the appendix by histology published since further definition of the various histologic subtypes by WHO. Since the data on appendiceal neuroendocrine tumor is still evolving, this study extended the comparison of survival outcomes to include appendiceal adenocarcinoma as well. While prior studies have suggested that atypical NET (GCC, NEC and MANEC) are more likely to present at more advanced stages, this study showed that most cases of GCC, MANEC and NEC were diagnosed at stages I and II. Appendiceal adenocarcinoma, on the other hand, presented mostly at stage IV. With respect to OS, atypical histologic subtypes of NET have worse outcome compared to TC. Although better OS was noted for GCC, NEC and MANEC when compared to adenocarcinoma, this benefit was lost in stage IV disease where adenocarcinoma recorded better 1- and 4-year OS. Prospective and randomized studies which provide granular details of treatment are needed to better define treatment algorithm for appendiceal NET.
Acknowledgements
We would like to thank the Surveillance Research Program (SRP) in National Cancer institute’s Division of Cancer Control and Population Sciences (DCCPS) for access to the SEER database that enabled this analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Since this research study utilized the SEER database which is a multi-institutional, de-identified cancer registry, informed consent or a review board approval is not necessary or applicable.
References
- Reznek RH. CT/MRI of neuroendocrine tumours. Cancer Imaging 2006;6:S163-77. [Crossref] [PubMed]
- Wang YH, Lin Y, Xue L, et al. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995–2012) in South China. BMC Endocr Disord 2012;12:30. [Crossref] [PubMed]
- Khan MS, Caplin ME. The use of biomarkers in neuroendocrine tumours. Frontline Gastroenterol 2013;4:175-81. [Crossref] [PubMed]
- Kim JY, Hong SM. Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts. Arch Pathol Lab Med 2016;140:437-48. [Crossref] [PubMed]
- Brathwaite S, Yearsley MM, Bekaii-Saab T, et al. Appendiceal Mixed Adeno-Neuroendocrine Carcinoma: A Population-Based Study of the Surveillance, Epidemiology, and End Results Registry. Front Oncol 2016;6:148. [Crossref] [PubMed]
- Brathwaite S, Rock J, Yearsley MM, et al. Mixed Adeno-neuroendocrine Carcinoma: An Aggressive Clinical Entity. Ann Surg Oncol 2016;23:2281-6. [Crossref] [PubMed]
- Ruoff C, Hanna L, Zhi W, et al. Cancers of the Appendix: Review of the Literatures. ISRN Oncol 2011;2011:728579. [Crossref] [PubMed]
- McCusker ME, Coté TR, Clegg LX, et al. Primary malignant neoplasms of the appendix. Cancer 2002;94:3307-12. [Crossref] [PubMed]
- La Rosa S, Marando A, Sessa F, et al. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel) 2012;4:11-30. [Crossref] [PubMed]
- Kelly KJ. Management of Appendix Cancer. Clin Colon Rectal Surg 2015;28:247-55. [Crossref] [PubMed]
- Pape UF, Perren A, Niederle B, et al. ENETS Consensus Guidelines for the Management of Patients with Neuroendocrine Neoplasms from the Jejuno-Ileum and the Appendix Including Goblet Cell Carcinomas. Neuroendocrinology 2012;95:135-56. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Misdraji J, Young RH. Primary epithelial neoplasms and other epithelial lesions of the appendix (excluding carcinoid tumors). Semin Diagn Pathol 2004;21:120-33. [Crossref] [PubMed]
- O’Donnell ME, Badger SA, Beattie GC, et al. Malignant neoplasms of the appendix. Int J Colorectal Dis 2007;22:1239-48. [Crossref] [PubMed]
- Roy P. Goblet cell carcinoid tumors of the appendix: An overview. World J Gastrointest Oncol 2010;2:251-8. [Crossref] [PubMed]