
Artonin E sensitizes TRAIL-induced apoptosis by DR5 upregulation and cFLIP downregulation in TRAIL-refractory colorectal cancer LoVo cells
Introduction
The global burden of colorectal cancer (CRC) indicates that CRC is one of the leading causes of illness and mortality. It is the third most commonly diagnosed cancer and the fourth cause of cancer-related death worldwide, published report showed approximately 1.4 million of new CRC diagnosed cases and 694,000 of deaths by CRC worldwide (1). Standard treatment for patients with CRC varies by tumor location and stage at diagnosis. Surgical removal is the most common treatment for early stage CRC. For patients with late-stage CRC is usually treated by anticancer drug alone or in combination with radiation therapy after surgery (2). The anticancer drug often triggers cellular cell death via apoptosis, particularly desirable treatment outcome that destroys cancer cells without inflammatory response and organelle dysfunction. However, the normal cells most likely to be damaged by anticancer drugs that may cause some unpleasant side effects. The specific target on cancer cells with minimal collateral damage to the normal cells is ideally strategy (3). The treatment of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is one of the ideal strategy and often used in CRC. The TRAIL interact with death receptors (DRs), including the DR TRAILR1 (also known as DR4) and TRAILR2 (also known as DR5), on the surface of cancer cells can trigger apoptotic cell death signaling through DR-mediated apoptosis pathway, also called “TRAIL-induced apoptosis”, without any harmful effects to normal cells (4-6). Unfortunately, some CRC cells can resist to TRAIL-induced apoptosis and remains a problem treatment of these CRC cells, so finding the strategies to enhancing TRAIL-induced apoptosis are required (7,8). In this study, The LoVo cell line was used as a model of TRAIL-refractory CRC cells. The LoVo cell line resist to TRAIL treatment through express lower level of DR5 than the other CRC cell lines significantly (9). Thus, artonin E was investigated its ability to induced DR5 expression in LoVo cell line in this study.
The phytochemical artonin E is one of known prenylated flavonoids isolated from plant genus Artocarpus spp., indigenous to tropical area in South-East Asia. In this study, artonin E was extracted from plant Artocarpus elasticus Reinw. ex Blume. Artonin E has been shown ability to inhibit growth of microorganism in broad spectrum activity (10,11), anti-inflammatory and anti-allergic activity via inhibit 5-lipoxygenase (12), and induce cytotoxic activity against various cancer cell lines, such as skin, lung, breast, and ovarian (13-17). Artonin E was reported that increase DR5 protein level in human gastric cancer AGS cell line (18). Our preliminary studies confirmed that artonin E could increase DR5 expression in TRAIL-refractory LoVo cell line as similar as AGS cell line. These preliminary studies suggested that artonin E has a potential use for combination with TRAIL treatment in TRAIL-refractory LoVo cell line. In this study, the mechanisms of artonin E to increase TRAIL-refractory LoVo cell death by combining with TRAIL were investigated. This indicated the potential application of artonin E as a synergistic agent for combining with TRAIL treatment in TRAIL-refractory CRC.
Methods
Chemical and antibodies
Artonin E [IUPAC name: 5-hydroxy-8,8-dimethyl-3-(3-methylbut-2-enyl)-2-(2,4,5-trihydroxyphenyl)pyrano(2,3-h)chromen-4-one] was obtained from Dr. Wilawan Mahabusarakum, Faculty of Science, Prince of Songkla University, Thailand in purified powder form. Recombinant TRAIL was purchased from Merck Millipore Corporation (Merck KGaA, Darmstadt, DE). Chemicals for cell viability assay including MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and Dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). The fluorescent Hoechst 33342 dye for fluorescence microscope observation was purchased from Fisher Scientific, Inc. (InvitrogenTM, Waltham, MA, USA). Reagent kit for mRNA extraction and cDNA synthesis were purchased from QIAGEN N.V. (QIAzolTM lysis reagent, Venlo, LI, NL) and Thermo Fisher Scientific, Inc. (RevertAidTM First Strand cDNA Synthesis Kit, FermentasTM, Waltham, MA, USA), respectively. Reagent kit for quantitative PCR was purchased from Thermo Fisher Scientific, Inc. (SYBR® Select Master Mix, Applied BiosystemsTM, Waltham, MA, USA). Antibodies (Abs) for Western blotting analysis including rabbit monoclonal Abs against DR5, beta-Actin, and anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA), and rabbit monoclonal Abs against cFLIP were obtained from Merck Millipore Corporation (Merck KGaA, Darmstadt, DE).
Cell culture
The human CRC cell line LoVo was obtained from the American Type Culture Collection (ATCC, Manassas, VA). It was cultured in RPMI 1640 medium (Gibco Life Technologies, Carlbad, CA, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Science, Little Chalfont, UK), 100 U/mL penicillin and 100 µg/mL streptomycin (GE Healthcare Life Science, Inc., Little Chalfont, UK) at 37 °C in a humidified 5% CO2 atmosphere routinely. The cultured cells in exponential phase of growth were used for assays.
Artonin E and TRAIL-mediated cytotoxicity evaluated by MTT assay
The cytotoxicity of artonin E and TRAIL were measured by cell proliferation analysis using MTT assay as described by Denizot and Lang (19). The LoVo cells were seeded into a 96-well plate (5×103 cells/well), and then culture medium containing 10, 50 and 100 ng/mL of TRAIL alone and combination with different artonin E concentrations at 10, 20, 30, 40 and 50 µM were added to each well and incubated for 24 h at 37 °C with 5% CO2. The control group was treated with 0.5% DMSO. After treatment, 0.5 mg/mL of MTT solution dissolved in culture medium was added and then incubated for 2 h at 37 °C with 5% CO2. After incubation with MTT, the solution was removed and 100 µL of DMSO was added to each well to dissolve the formazan crystals, obtained from viable cells, and the absorbance at 540 nm was quantified on EpochTM Microplate Spectrophotometer and analyzed by Gen5TM Data Analysis Software (BioTek, CA, USA).
Evaluation of artonin E and TRAIL-induced apoptosis by chromatin condensation observation
Chromatin condensation, a character of apoptosis, was observed by cell staining with a fluorescent Hoechst 33342 dye as described by Oberhammer et al. (20). The LoVo cells were seeded into a 12-well plate (8×104 cells/well), and then culture medium containing 10, 50 and 100 ng/mL of TRAIL alone and combination with different artonin E concentrations 10, 20, 30, 40 and 50 µM were added to each well and incubated for 24 h at 37 °C with 5% CO2. The control group was treated with 0.5% DMSO. After treatment, the treated cells were washed and fixed with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were washed and then stained with 5 µg/mL of Hoechst 33342 solution for 15 min. After staining, the stained cells were washed and observed under a fluorescence microscope IX73 model (Olympus, Tokyo, Japan) with U-MWU2 mirror units for ultraviolet excitation.
mRNA expression analysis of artonin E-treated LoVo cell line by reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression was determined by RT-qPCR. The LoVo cells were seeded into a 12-well plate (8×104 cells/well), and then culture medium containing different artonin E concentrations 10, 20, 30, 40 and 50 µM were added to each well and incubated for 24 h at 37 °C with 5% CO2. The control group was treated with 0.5% DMSO. After treatment, the whole cells were collected and transferred into step of RNA extraction by QIAzolTM lysis reagent (Qiagen N.V.) and then followed step of cDNA synthesis by reverse transcription according RevertAidTM First Strand cDNA Synthesis Kit (FermentasTM, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 2 µg of total RNA of each sample. These cDNA were quantified by step of quantitative PCR using SYBR Select Master Mix (Applied BiosystemsTM, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The primers used for amplification were: DR5 (forward 5'-CACCAGGTGTGATTCAGGTG-3' and reverse 5'-TACGGCTGCAACTGTGACTC-3'), cFLIP (forward 5'- ATTGCATTGGCAATGAGACAGAGC-3' and reverse 5'- TCGGTGCTCGGGCATACAGG-3'), and GAPDH (forward 5'-AGGTCGGAGTCAACGGATTT-3' and reverse 5'-TAGTTGAGGTCAATGAAGGG-3'). The PCR amplification was analyzed by CFX96 TouchTM Real-Time PCR Detection System with CFX ManagerTM Software (Bio-Rad Laboratories, Inc., CA, USA). All steps were performed according to the manufacturer’s instructions.
Protein expression analysis of artonin E-treated LoVo cell line by Western blot
The protein expression was determined by Western blot. The LoVo cells were seeded into a 6-well plate (2×105 cells/well), and then culture medium containing different artonin E concentrations 10, 20, 30, 40 and 50 µM were added to each well and incubated for 24 h at 37 °C with 5% CO2. The control group was treated with 0.5% DMSO. After treatment, the whole cells were collected and lysed with RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 250 mM NaCl, 0.5% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS) supplemented with Roche® cOmplete Protease Inhibitor Cocktail Tablets (Roche Diagnostics, Switzerland), and homogenized by sonication. The extracted protein concentration was determined by using Bio-Rad® protein assay (Bio-Rad Laboratories, USA) which based on the method of Bradford’s with bovine serum albumin (BSA) as standard protein. Ten microgram of cell lysate protein was separated on 12.5% acrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Merck Millipore Corporation, Merck KGaA, Darmstadt, DE). The transferred membrane was blocked with 5% skimmed-milk in TBS-Tween buffer for 1 h at room temperature and then incubated with the appropriate primary antibody concentration (1:1,000 dilution for DR5; 1:1,000 dilution for cFLIP; and 1:1,000 for beta-actin) overnight at 4 °C. The processed membranes were subsequently washed in TBS-Tween buffer and then incubated at room temperature with anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibodies (1:10,000 dilutions). After the membranes were exposed to the secondary antibodies, the signals were developed using Immobilon™ Western Chemiluminescent HRP substrate (Merck Millipore) and detected using a chemiluminescent imaging system (GeneGnome gel documentation; Synoptics Ltd., Cambridge, UK). Intensity analysis of protein bands were performed using ImageJ software (National Institutes of Health, USA).
Statistical analysis
To compare the data from different groups, Microsoft Excel version 2010 software (Microsoft Corporation, Redmond, WA, USA) was used to analyze the data by Student’s t-test. All data presented were obtained from at least three independent experiments and were presented as mean ± standard deviation (SD). A P value less than 0.05 were considered to indicate a statistically significant difference.
Results
Artonin E sensitized TRAIL-induced apoptosis in TRAIL-refractory LoVo cells
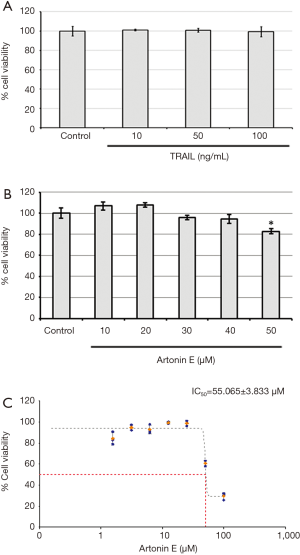
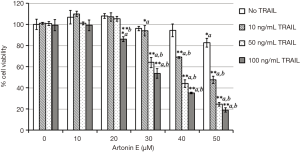
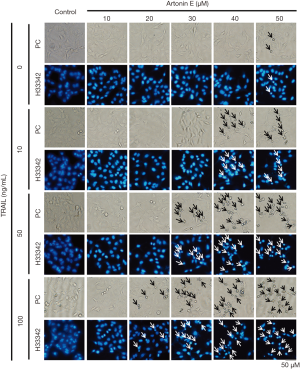
In this study, we further proved that LoVo cells were resistant to TRAIL and insensitive to artonin E by MTT assay. As results shown in Figure 1, the treatment of TRAIL alone in LoVo cells at concentration 10, 50 and 100 ng/mL showed more than 80% cell viability, this result indicated that the LoVo cells showed resistance to TRAIL-induced cell death. Likewise, the treatment of artonin E alone in LoVo cells at concentration of 10, 20, 30, 40 and 50 µM showed more than 80% cell viability indicating that the LoVo cells insensitive to artonin E triggered cell death. Cell viability curve of artonin E on LoVo cells showed cytotoxicity with high IC50 value at 55.065±3.833 µM, however, we found that combined treatment of artonin E and TRAIL increase cytotoxicity in TRAIL-refractory LoVo cells in a dose dependent manner as shown in Figure 2. We confirmed the effect of artonin E on apoptosis induction using Hoechst 33342 staining to assess chromatin condensation as shown in Figure 3. Treatment of LoVo cells with combination of artonin E and TRAIL increased chromatin condensation LoVo cells suggesting that the treatment of artonin E sensitize TRAIL-induced apoptosis in LoVo cells. The effect of artonin E on LoVo cells beneficial for sensitizing TRAIL on LoVo cells were investigated in the next steps to assess apoptosis pathway.
Artonin E enhances TRAIL-induced apoptosis through DR5 upregulation and cFLIP downregulation
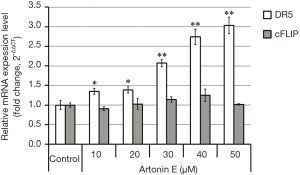
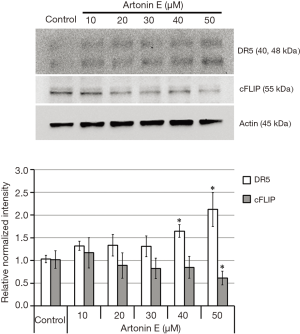
TRAIL triggers cell death signal via the binding of TRAIL to DR5, resulting in the subsequent caspase-dependent apoptosis induction. As shown in Figure 4, the treatment of artonin E at various concentrations showed that artonin E enhances mRNA of DR5 expression significantly, but no different in mRNA of cFLIP expression. However, the results of Western blotting analysis as shown in Figure 5 indicated that protein expression level of DR5 corresponded with mRNA expression, but the level of cFLIP was significantly decreased at the highest concentration of artonin E treatment. However, the expressed mRNA may regulate post-transcriptional modification before translation initiation process and the expressed proteins are molecular targets directly. Thus, these results implied that DR5 upregulation and cFLIP downregulation resulting from artonin E treatment and contributed to enhance TRAIL sensitization.
Discussion
TRAIL is a functional ligand protein that induces apoptosis process through death-receptor mediated pathway. It has a potential use in cancer therapy as it showed selective toxicity in cancer cells but less toxic in normal cells (4-6). In present, the usage of TRAIL as anticancer agent has been tested in phase I and II trials in patients with advanced cancer (21). However, the treatment of TRAIL alone is probably not practicable procedure because several cancer cells, especially some highly malignant tumors, are resistant to apoptosis induction by TRAIL (22). Thus, the combination treatment of TRAIL and anticancer agents is essential use in TRAIL-refractory cancer therapy. Therefore, we also analyzed the effect of cytotoxic agent artonin E’s ability to enhance cytotoxicity and apoptosis in combination treatment with TRAIL, indicating potential use of artonin E for TRAIL-refractory cancer therapy.
CRC remains a significant problem worldwide, especially TRAIL refractory CRC. Standard anticancer drugs may cause serious side effects suggested that the combined use of non-toxic dose cytotoxic agents and TRAIL was investigated. LoVo cell is a human CRC cell line that was reported to be resistant to TRAIL (9). Although some natural compounds, such as laminarin goniothalamin, etc., were found to be able to enhance the anticancer effects of LoVo cells to TRAIL (23,24). Artonin E was reported to trigger DR5 in human gastric adenocarcinoma AGS cells, then resulting to enhance TRAIL-induced apoptosis (18), but the effect of artonin E on TRAIL-refractory LoVo cells was not reported. Then, we focused on investigating the potential use of artonin E for sensitizes TRAIL-induced apoptosis in LoVo cells.
In this study, we investigate the expression pattern of major beneficial mediators, including DR5 upregulation and cFLIP downregulation which were able to trigger TRAIL-induced apoptosis, at mRNA level and protein level (25). The results of mRNA expression profiles indicated that artonin E initiated the increased DR5 mRNA expression with the lowest concentration of artonin E used, while mRNA expression of cFLIP was not changed. However, protein expression level was not corresponded. The DR5 protein expression was slightly increased and significantly increased at the high artonin E concentration while the protein expression of cFLIP was significantly decreased at the highest artonin E concentration. The protein level may not be consistent with mRNA level because there are many complicated and varied post-transcriptional mechanisms involved in turning these mRNA into protein (26). Thus, we mainly consider the beneficial effects of artonin E on sensitize TRAIL-induced apoptosis through the protein expression level in this case. The DR5 protein directly target to TRAIL resulted in triggering TRAIL-induced apoptosis (27) while the cFLIP protein directly target to Fas-associated protein with death domain (FADD) and/or caspase-8 and forms an apoptosis inhibitory complex (AIC) to prevent the downstream TRAIL-induced apoptosis cascade (28,29). So, the increase protein level of DR5 and the decrease protein level of cFLIP by artonin E were mainly caused by sensitization of TRAIL-induced apoptosis in TRAIL-refractory LoVo cells.
Conclusions
In this work, the obtained results reveal that artonin E enhanced TRAIL ability to be selectively cytotoxic to TRAIL-refractory CRC LoVo cells through both DR5 up-regulation and cFLIP down-regulation. These effect was similar to other compounds sensitize TRAIL-induced apoptosis, such as goniothalamin (23), vitamin E analog (α-TEA) (30), paxilline (31), and silibinin (32). In addition, this is the first report showed the effect of artonin E on cFLIP down-regulation. The results suggested that combined treatment of TRAIL and artonin E provides a possible therapeutic application for treatment of CRC that are resistant to TRAIL.
Acknowledgements
Funding: This work was supported by research scholarship (RRI 61/2558) from Research Institute of Rangsit University, Pathum Thani, Thailand.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Akhtar R, Chandel S, Sarotra P, et al. Current status of pharmacological treatment of colorectal cancer. World J Gastrointest Oncol 2014;6:177-83. [Crossref] [PubMed]
- Baig S, Seevasant I, Mohamad J, et al. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis 2016;7:e2058. [Crossref] [PubMed]
- Mahalingam D, Szegezdi E, Keane M, et al. TRAIL receptor signaling and modulation: Are we on the right TRAIL? Cancer Treat Rev 2009;35:280-8. [Crossref] [PubMed]
- Kichev A, Rousset CI, Baburamani AA, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. J Biol Chem 2014;289:9430-9. [Crossref] [PubMed]
- Holland PM. Death receptor agonist therapies for cancer, which is the right TRAIL? Cytokine Growth Factor Rev 2014;25:185-93. [Crossref] [PubMed]
- Van Geelen CM, de Vries EG, de Jong S. Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat 2004;7:345-58. [Crossref] [PubMed]
- Trarbach T, Moehler M, Heinemann V, et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer 2010;102:506-12. [Crossref] [PubMed]
- Galligan L, Longley DB, McEwan M, et al. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther 2005;4:2026-36. [Crossref] [PubMed]
- Kuete V, Ango PY, Fotso GW, et al. Antimicrobial activities of the methanol extract and compounds from Artocarpus communis (Moraceae). BMC Complement Altern Med 2011;11:42. [Crossref] [PubMed]
- Zajmi A, Mohd Hashim N, Noordin MI, et al. Ultrastructural study on the antibacterial activity of artonin E versus streptomycin against Staphylococcus aureus strains. PLoS One 2015;10:e0128157. [Crossref] [PubMed]
- Bellik Y, Boukraâ L, Alzahrani HA, et al. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules 2012;18:322-53. [Crossref] [PubMed]
- Wongpankam E, Chunhacha P, Pongrakhananon V, et al. Artonin E mediates MCL1 down-regulation and sensitizes lung cancer cells to anoikis. Anticancer Res 2012;32:5343-51. [PubMed]
- Plaibua K, Pongrakhananon V, Chunhacha P, et al. Effects of artonin E on migration and invasion capabilities of human lung cancer cells. Anticancer Res 2013;33:3079-88. [PubMed]
- Etti I, Abdullah R, Hashim NM, et al. Artonin E and structural analogs from Artocarpus species abrogates estrogen receptor signaling in breast cancer. Molecules 2016;21:E839. [Crossref] [PubMed]
- Etti IC, Rasedee A, Hashim NM, et al. Artonin E induces p53-independent G1 cell cycle arrest and apoptosis through ROS-mediated mitochondrial pathway and livin suppression in MCF-7 cells. Drug Des Devel Ther 2017;11:865-79. [Crossref] [PubMed]
- Etti IC, Abdullah R, Kadir A, et al. The molecular mechanism of the anticancer effect of Artonin E in MDA-MB 231 triple negative breast cancer cells. PLoS One 2017;12:e0182357. [Crossref] [PubMed]
- Toume K, Habu T, Arai MA, et al. Prenylated flavonoids and resveratrol derivatives isolated from Artocarpus communis with the ability to overcome TRAIL resistance. J Nat Prod 2015;78:103-10. [Crossref] [PubMed]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89:271-7. [Crossref] [PubMed]
- Oberhammer FA, Hochegger K, Fröschl G, et al. Chromatin condensation during apoptosis is accompanied by degradation of Lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol 1994;126:827-37. [Crossref] [PubMed]
- Stuckey DW, Shah K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol Med 2013;19:685-94. [Crossref] [PubMed]
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 2005;12:228-37. [Crossref] [PubMed]
- Sophonnithiprasert T, Nilwarangkoon S, Nakamura Y, et al. Goniothalamin enhances TRAIL-induced apoptosis in colorectal cancer cells through DR5 upregulation and cFLIP downregulation. Int J Oncol 2015;47:2188-96. [Crossref] [PubMed]
- Ji CF, Ji YB. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol Lett 2014;7:1728-32. [Crossref] [PubMed]
- Shlyakhtina Y, Pavet V, Gronemeyer H. Dual role of DR5 in death and survival signaling leads to TRAIL resistance in cancer cells. Cell Death Dis 2017;8:e3025. [Crossref] [PubMed]
- Kang Z, Sun SY, Cao L. Activating death receptor DR5 as a therapeutic strategy for rhabdomyosarcoma. ISRN Oncol 2012;2012:395952. [Crossref] [PubMed]
- Greenbaum D, Colangelo C, Williams K, et al. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 2003;4:117. [Crossref] [PubMed]
- Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol 2012;34:176-84. [PubMed]
- Hughes MA, Powley IR, Jukes-Jones R, et al. Co-operative and hierarchical binding of c-FLIP and caspase-8: A unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell 2016;61:834-49. [Crossref] [PubMed]
- Yu W, Tiwary R, Li J, et al. α-TEA induces apoptosis of human breast cancer cells via activation of TRAIL/DR5 death receptor pathway. Mol Carcinog 2010;49:964-73. [Crossref] [PubMed]
- Kang YJ, Kim IY, Kim EH, et al. Paxilline enhances TRAIL-mediated apoptosis of glioma cells via modulation of c-FLIP, survivin and DR5. Exp Mol Med 2011;43:24-34. [Crossref] [PubMed]
- Son YG, Kim EH, Kim JY, et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res 2007;67:8274-84. [Crossref] [PubMed]


