
Evaluating treatment protocols for rectal squamous cell carcinomas: the Duke experience and literature
Introduction
Colorectal cancer is the third most common cancer and the third leading cause of cancer deaths in both men and women in the United States (1). Within the rectum, over 90% of malignancies are adenocarcinomas (2). In contrast, rectal squamous cell carcinomas (SCC) represent only 0.1% to 0.3% of all colorectal cancers, with approximately 200 cases reported to date (3-5). The first case of SCC in the rectum was described by Raiford in 1933 (6), and Williams later proposed the following criteria for diagnosis (7): (I) metastatic SCC must be excluded; (II) the tumor must not have a squamous lined fistulous tract; (III) the tumor cannot represent proximal extension of SCC of the anus, and (IV) histologic confirmation. Though histologically similar to anal SCC and anatomically identical to rectal adenocarcinomas, rectal SCC is a rare and unique malignancy for which evidence and clinical consensus surrounding treatment are lacking.
Treatment for rectal SCC has evolved over time, from a surgical approach as used in rectal adenocarcinomas and transitioning to primary chemoradiotherapy (CRT) similar to current management of anal SCC. Although more recent data support favorable outcomes with high-dose CRT as the primary intervention for rectal SCC, evidence is still limited and derived primarily from case reports, case series, one large population-based study (1), and one meta-analysis (3). As large randomized prospective trials are unrealistic in the setting of this rare malignancy, this study evaluates an institutional experience in eight patients and reviews the existing literature to help guide future management approaches.
Methods
This retrospective study compared various treatments for patients with rectal SCC treated at Duke University Medical Center from January 1, 1980 through December 31, 2016. Patients were identified through Duke Enterprise Data Unified Content Explorer (DEDUCE) as well as paper and electronic medical records in the Department of Radiation Oncology. Forty-seven charts were identified based on ICD codes (ICD-9: 154, 154.0, 154.1, 153.3, 153.8, and 153.9; ICD-10: C20.0), histology, and keywords (rectal SCC) within the study time period, anticipating that many of these charts included patients with rectal adenocarcinoma as well as SCC of non-rectal origin. This protocol did not involve prospective enrollment of subjects, and consent was obtained through a Waiver or Alteration of Consent and HIPAA Authorization and Decedent Research Notification.
Patients over age 18 with nonmetastatic rectal SCC with histologic confirmation were included in this analysis. The primary tumor was required to clearly be a rectal-based tumor, with no involvement of the anal canal and all disease above the anorectal ring and/or greater than 4 cm from the anal verge. Patients with M1 disease as defined by 2010 AJCC staging system and with disease originating in the anal canal and extending proximally into the rectum were excluded.
Collected variables included patient characteristics [gender, age at diagnosis, date of diagnosis, date of first contact, human immunodeficiency virus (HIV) status], tumor characteristics (tumor size, tumor histology, tumor grade, tumor distance from anal verge, rectal circumference involved), treatment details [date of chemotherapy (CT), CT regimen and toxicities, radiation therapy (RT) technique and dosage, dates, RT completion, toxicities from RT as determined by the RTOG/EORTC Radiation Toxicity Grading Scale, toxicities from CT as determined by the ECOG Common Toxicity Criteria, date of surgical treatment, surgical approach, residual disease, postoperative complications], and outcomes (locoregional control, distant recurrence, disease-free survival, overall survival, age at death, cause of death, follow up duration, colostomy status).
Due to small sample size, all statistical analyses are descriptive. For our systematic review, a comprehensive search of PubMed was performed (search terms: squamous cell carcinoma and rectal cancer), along with the reference list of selected articles reviewed to ensure all relevant publications were reviewed. The search spanned from 1933 to March 2018. A qualitative analysis was performed to examine patient diagnosis, treatment, and outcome.
Results
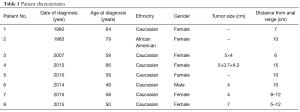
Eight patients with nonmetastatic rectal SCC were evaluated at Duke University Medical Center from 1980 to 2016. Patient and tumor characteristics are presented in Table 1. The majority of patients were female (87.5%). One patient was HIV positive. Tumor size varied from 3.7 to 5.6 cm, but was not reported for half of the patients. The distance from the anal verge ranged from 5 to 15 cm. The median age was 58.5 years (range, 49–79 years).
Full table
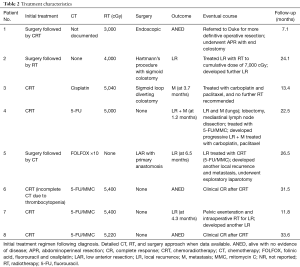
Treatment characteristics are summarized in Table 2. Three patients underwent initial, curative attempt surgery followed by adjuvant RT, CT, or CRT. There were a range of surgical approaches, including endoscopic resection, Hartmann’s procedure, and low anterior resection (LAR). Two of the three surgical patients required colostomy. Adjuvant RT dose ranged from 3,000 to 4,000 cGy. Adjuvant CT regimens varied and were not adequately documented in one of the cases. Of the reported cases, none used a 5-fluorouracil (FU)/mitomycin C (MMC) regimen. With follow-up ranging from 7.1 to 31.5 months, one patient was alive with no evidence of disease and two patients developed local/regional recurrence (one developed and expired from liver metastases). These two patients received additional treatment: one received CRT and the other received additional RT. Both ultimately progressed and expired.
Full table
Five patients were initially managed nonoperatively with definitive CRT (one had initial diverting colostomy). One patient received 5-FU, one cisplatin and three 5-FU/MMC. One patient received 5,000 cGy, another 5,040 cGy, another 5,220 cGy, and two patients 5,400 cGy. Of these, one experienced grade 2 acute dermatitis related to radiation. With follow-up duration in these patients ranging from 11.8 to 33.6 months, one patient experienced local recurrence, one developed liver metastases and another both local recurrence and distant metastases. Of these, two patients were treated with surgery, with additional CT in one and intraoperative RT in another. The other received palliative chemotherapy. The fourth and fifth patients achieved complete response on imaging following initial management with CRT (follow-up of 31.5 and 33.6 months) and currently both remain disease-free.
Discussion
Although histologically similar to anal SCC and anatomically identical to rectal adenocarcinomas, rectal SCC represents a unique malignancy that is distinct in pathogenesis and epidemiology. While the pathogenesis of rectal SCC still requires further characterization, several hypotheses and associations have been proposed, including proliferation of stem cells capable of multidirectional differentiation, chronic inflammatory processes such as ulcerative colitis, infections ranging from schistosomiasis to human papillomavirus, radiation exposure, and squamous differentiation from an underlying adenoma or adenocarcinoma (5,8). Despite the lack of robust epidemiological data, several retrospective series have described trends and suggest that rectal SCC tends to occur more frequently in women (5), as was the case in the present analysis, and is more often presents with advanced disease (9) when compared with rectal adenocarcinomas.
Given its rarity, evidence and clinical consensus on the optimal treatment of rectal SCC are lacking. Despite the differences in anal SCC and rectal adenocarcinoma, both staging and management of rectal SCC have been derived from treatment approaches of these better-characterized malignancies. The standard of care for locally advanced rectal adenocarcinomas is neoadjuvant, short-course RT or long-course, fluoropyrimidine-based CRT, followed by surgical resection (10,11). The surgical approach depends on tumor characteristics (including staging, location, depth of invasion, local and distant metastases) as well as patient factors (including comorbidities and body habitus) (10,12). Historically, rectal SCC patients were managed similarly to rectal adenocarcinomas, primarily relying on LAR or abdominoperineal resection (APR), which are associated significant morbidity (13–46%) (13) and mortality (1–7%) (13,14).
Like rectal cancer, anal SCC was historically managed by surgery (11). However, the treatment of anal SCC was revolutionized beginning in 1974 by the Nigro protocol consisting of 3,000 cGy RT given over 3 weeks, combined with 1,000 mg/m2/day 5-FU given as a continuous infusion over days 1 through 4 and repeated on days 29 through 32, with 10 mg/m2 MMC delivered on days 1 and 29 (15). Multiple studies have since demonstrated the benefit of the combined CRT approach using 5-FU/MMC, as compared to RT alone of other chemotherapy combinations (16-19). This combined-modality approach results in long-term local control, colostomy-free and overall survival in the majority of anal cancer patients. With these results confirmed by randomized trials, CRT is the accepted standard of care for anal SCCs, with surgery reserved for salvage.
Reflecting this shift in anal SCC treatment paradigms, recent reports have increasingly focused on CRT as the primary therapeutic intervention for rectal SCC patients, similarly reserving surgery for salvage. Early experiences showed mixed results but more recently, CRT as the primary intervention has consistently shown outcomes that are more promising. Similar to anal SCC treatment guidelines, most case reports and series of rectal SCC have used a 5-FU-based CRT regimen. Appropriate RT approaches include treatment of the tumor, mesorectum, pre-sacral nodes, and internal iliac nodal basins as target, with most authors recommending a dose between 5,400–6,000 cGy (4).
In the present analysis, most patients treated initially with surgery had suboptimal disease control. Although most patients in our series treated with combined CRT also experienced disease progression, two patients had a complete tumor response to treatment without need for salvage surgery or additional treatment. Though our analysis is limited by small numbers, this analysis and others suggest definitive CRT is the preferred treatment approach to rectal SCC.
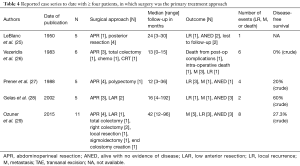
This conclusion is supported by other reports in the literature. We summarize the largest case series comparing primary CRT vs. surgery in Tables 3,4. In the largest case series to date of rectal SCC by Loganadane et al. in France (4), 23 patients were treated at two institutions from 1992 to 2013. Twenty-one received CRT while two patients received pre-operative CRT followed by planned surgery. Radiation dose ranged from 3,600 to 4,500 cGy, with a boost dose of 1,500 to 2,300 cGy. With a median follow-up of 85 months, the clinical complete response rate was 83% and the 5-year disease-free, colostomy-free and overall survival rates were 81%, 65% and 86%, respectively. Based on the high local control rate and prolonged survival in these patients, the authors recommended rectal SCC be treated similarly to anal SCC. The authors also raised the issue of the best method of evaluating residual disease, including imaging modality and optimal timing of surgery, if indicated.

Full table
Full table
A Surveillance, Epidemiology, and End Results (SEER) registry population-based analysis of 999 rectal SCC patients treated from 1998 to 2011 was performed (1). Their analysis demonstrated that rectal SCC patients, as compared to rectal adenocarcinoma patients over the same time period, were more commonly female, were associated with larger tumors of higher grade, and were more often treated with radiotherapy than surgery. Surgery did not appear to improve survival, and RT had proportionally greater benefits in rectal SCC as compared to adenocarcinoma patients. These authors recommended non-surgical, RT-based treatment in these patients. Interestingly, this study also notes that the number of rectal SCC diagnoses are increasing over time and that the evidence regarding optimal treatment protocols is limited, highlighting the need for further research. This type of study is limited by its retrospective nature, lack of chemotherapy records, potential selection bias, and potential misdiagnosis of anal SCC with extension into the rectum.
The largest literature review to date by Guerra et al. in 2016 (3) consists of a systematic review of Ovid MEDLINE articles spanning from 1946 to 2015, which identified 487 articles. Of these, 79 were included in the qualitative review and 63 in the quantitative analysis. In this review, pooled overall survival was 86% in patients with initial CRT, compared to 48% in patients treated with surgery initially. The authors summarize that while there is no consensus on the optimal treatment approach, there has been a recent shift from surgery towards CRT as primary therapy, with resultant improved local control. Limitations of this study include heterogeneity of case reports.
Given our limited sample sizes and literature primarily limited to smaller case studies and series, it is difficult to reach a definitive conclusion from this data. However, it raises important questions about the role of initial surgery in this disease, given the high rates of local failure and generally poor outcomes. It also raises questions for how to optimize CRT as the initial treatment approach for rectal SCC, including CT regimen, RT approach and targets, optimal evaluation of residual tumor status, and appropriate monitoring of treatment response, particularly when some candidates will require salvage surgery.
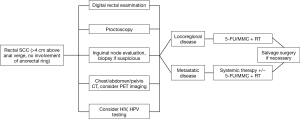
In summary, the optimal management and evaluation of tumor response for rectal SCC remains unknown. As randomized trials are challenging given the rarity of this malignancy, evidence based on case reports and case series contribute to our understanding of the epidemiology and therapeutic management. Treatment of rectal SCC has evolved from upfront surgery to definitive CRT as initial therapy. However, there is a perception that treatment for rectal SCC should involve primary surgery even in contemporary series, despite the growing evidence of improved outcomes achieved with CRT alone (20). Despite this, the available data, including the present series as well as a review of the existing literature, indicate definitive CRT is preferred to surgery based on improved clinical outcomes, sphincter preservation and morbidity profile. Figure 1 shows a proposed treatment algorithm for these patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest:All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2018.11.02). CGW serves as an unpaid editorial board member of Journal of Gastrointestinal Oncology from Jan 2019 to Dec 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Duke University IRB (No. 00083063). This protocol did not involve prospective enrollment of subjects, and consent was obtained through a Waiver or Alteration of Consent and HIPAA Authorization and Decedent Research Notification.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiu MS, Verma V, Bennion NR, et al. Comparison of outcomes between rectal squamous cell carcinoma and adenocarcinoma. Cancer Med 2016;5:3394-402. [Crossref] [PubMed]
- Kulaylat AS, Hollenbeak CS, Stewart DB Sr. Squamous Cancers of the Rectum Demonstrate Poorer Survival and Increased Need for Salvage Surgery Compared With Squamous Cancers of the Anus. Dis Colon Rectum 2017;60:922-7. [Crossref] [PubMed]
- Guerra GR, Kong CH, Warrier SK, et al. Primary squamous cell carcinoma of the rectum: An update and implications for treatment. World J Gastrointest Surg 2016;8:252-65. [Crossref] [PubMed]
- Loganadane G, Servagi-Vernat S, Schernberg A, et al. Chemoradiation in rectal squamous cell carcinoma: Bi-institutional case series. Eur J Cancer 2016;58:83-9. [Crossref] [PubMed]
- Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol 2009;15:4380-6. [Crossref] [PubMed]
- Raiford TS. Epitheliomata of the lower rectum and anus. Surg Gynecol Obstet 1933;57:21-35.
- Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol 1979;129:139-47. [Crossref] [PubMed]
- Copur S, Ledakis P, Novinski D, et al. Squamous cell carcinoma of the colon with an elevated serum squamous cell carcinoma antigen responding to combination chemotherapy. Clin Colorectal Cancer 2001;1:55-8. [Crossref] [PubMed]
- Lafreniere R, Ketcham AS. Primary squamous carcinoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum 1985;28:967-72. [Crossref] [PubMed]
- Copur MS, Schneider S, Mleczko K, et al. Woman With Rare Cause of Rectal Bleeding. Oncology (Williston Park) 2018;32:28-31. [PubMed]
- Czito BG, Meyer J. Radiation therapy in anal and rectal cancer. Surg Oncol Clin N Am 2013;22:525-43. [Crossref] [PubMed]
- Rasheed S, Yap T, Zia A, et al. Chemo-radiotherapy: an alternative to surgery for squamous cell carcinoma of the rectum--report of six patients and literature review. Colorectal Dis 2009;11:191-7. [Crossref] [PubMed]
- Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000;356:93-6. [Crossref] [PubMed]
- Arenas RB, Fichera A, Mhoon D, et al. Total mesenteric excision in the surgical treatment of rectal cancer: a prospective study. Arch Surg 1998;133:608-11; discussion 611-2. [Crossref] [PubMed]
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. [Crossref] [PubMed]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. [Crossref] [PubMed]
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol 2013;14:516-24. [Crossref] [PubMed]
- Clark J, Cleator S, Goldin R, et al. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care? Eur J Cancer 2008;44:2340-3. [Crossref] [PubMed]
- Sturgeon JD, Crane CH, Krishnan S, et al. Definitive Chemoradiation for Squamous Cell Carcinoma of the Rectum. Am J Clin Oncol 2017;40:163-6. [Crossref] [PubMed]
- Musio D, De Felice F, Manfrida S, et al. Squamous cell carcinoma of the rectum: The treatment paradigm. Eur J Surg Oncol 2015;41:1054-8. [Crossref] [PubMed]
- Nahas CS, Shia J, Joseph R, et al. Squamous-cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum 2007;50:1393-400. [Crossref] [PubMed]
- Peron J, Bylicki O, Laude C, et al. Nonoperative management of squamous-cell carcinoma of the rectum. Dis Colon Rectum 2015;58:60-4. [Crossref] [PubMed]
- LeBlanc LJ, Buie LA, Dockerty MB. Squamous-cell epithelioma of the rectum. Ann Surg 1950;131:392-9. [Crossref] [PubMed]
- Vezeridis MP, Herrera LO, Lopez GE, et al. Squamous-cell carcinoma of the colon and rectum. Dis Colon Rectum 1983;26:188-91. [Crossref] [PubMed]
- Prener A, Nielsen K. Primary squamous cell carcinoma of the rectum in Denmark. APMIS 1988;96:839-44. [Crossref] [PubMed]
- Gelas T, Peyrat P, Francois Y, et al. Primary squamous-cell carcinoma of the rectum: report of six cases and review of the literature. Dis Colon Rectum 2002;45:1535-40. [Crossref] [PubMed]
- Ozuner G, Aytac E, Gorgun E, et al. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis 2015;30:127-30. [Crossref] [PubMed]


