
What to expect with major vascular reconstruction during Whipple procedures: a single institution experience and literature review
Introduction
Pancreatic adenocarcinoma is a highly lethal disease that has a 5-year survival as low as 6% in the United States (1,2). Most patients with pancreatic cancer are asymptomatic until the disease has reached an advanced stage often limiting potential curative resection (2). In addition, the tumor biology of pancreatic adenocarcinomas leads to early recurrence and metastasis with resistance to conventional neo-adjuvant and adjuvant therapies (chemotherapy, radiotherapy) (1). Early surgical resection remains to be regarded as the only treatment that potentially leads to increased patient survival, and cure (3-8). A common surgery for resection of pancreatic head adenocarcinoma is a pancreaticoduodenectomy (PD), or Whipple procedure (3-8), which involves resecting the head of the pancreas, duodenum, gallbladder, and bile duct (9). However, when the surgical margin is positive for cancer (R1 resection); survival rates remain very poor, thus putting an emphasis on accurate preoperative diagnosis and surgical interventions that fully removes the disease (10). In order to achieve a negative margin (R0 resection), resection of vessels [superior mesenteric vein (SMV), portal vein (PV), and hepatic artery] may be deemed necessary. Because the PV, SMA, and hepatic arteries may be involved a vascular resection during PD often requires secondary vascular reconstruction of the resected vessels. Literature has debated the feasibility of vascular resection and the secondary reconstruction during Whipple procedures. Multiple single-center series reports argue that vascular resection/reconstruction is a safe and feasible procedure during PD in an attempt to obtain negative margins (10-22). However, multiple studies, including those arguing for the feasibility and safety of the procedure, have data showing that vascular reconstruction secondary to resection leads to decreased survival rates (1-, 3-, 5-year) and increased postoperative complications compared to PD without vascular intervention (3,12,16,23,24). These inconsistencies call for further analysis of the outcomes associated with PD involving vascular reconstruction, and their impact on patient care. The ability to accurately inform a patient with pancreatic carcinoma during the decision making process is critical to holistic, patient-orientated healthcare. Additionally, the process of engaging with the patient, and discussing treatment options and postoperative expectations, is especially critical for older patients undergoing high-risk surgeries (i.e., PD with vascular reconstruction) (25).
The aim of this study is to report our 8-year experience of Whipple procedures involving vascular reconstruction and to review relevant literature to further evaluate expectant outcomes of the surgery in order to increase patient awareness during the decision making progress.
Methods
A retrospective review of the patients who underwent PD, pylorus-preserving pancreaticoduodenectomy (PPPD), and total pancreatectomy (TP) after Whipple procedures, between January 2010 and December 2017 at the Mayo Clinic Jacksonville, Florida, was performed using data collected from a Mayo Clinic Institutional Review Board (IRB)-approved prospective database (IRB 09-00-3940). Informed consent was waived by the IRB as this study was deemed minimal risk to patients. Patients with clinical, radiologic, and final pathologic confirmation of pancreatic adenocarcinomas, who underwent surgical intervention involving an open or laparoscopic (including intraoperative conversion) Whipple procedure, were included in the study. Patient information, including demographics, clinical history, symptoms, previous surgeries, and tumor pathologies, was collected.
Patients were divided into two separate groups: those who underwent additional major vascular reconstruction following resection of vessels (SMV, PV, and hepatic artery) during a Whipple procedure, and those who did not require additional vascular manipulation. The two groups were compared in terms of demographics, intraoperative characteristics (estimated blood loss, amount of blood transfused, operative time, vascular surgeon involvement, resection margins) type of major vascular reconstruction [primary anastomosis, interposition polytetrafluoroethylene (PTFE) graft, patch, and complex], type of vessel resected/reconstructed (PV, SMV, hepatic artery, etc.) and postoperative outcomes (hospital stay, intensive care unit (ICU) stay, readmission rates, 30-day survival, 1-year survival). The patients were followed postoperatively to determine if readmission was due to complications involved with the vascular reconstruction (thrombosis, etc.) and if continued treatment/follow-up was required for those complications. Lateral renorrhaphy was not considered a major vascular reconstruction in this study.
Statistical analysis was performed using both a two-sided Fisher’s exact test and an unpaired t-test for categorical data. Statistical significance was defined as a P value of less than 0.05.
Results
Preoperative characteristics
Demographics, symptoms, and comorbidities of patients undergoing conventional Whipple (−) and Whipple involving vascular reconstruction (+) data are presented in Table 1. Of the 29 patients who underwent Whipple with vascular reconstruction, 12 (41.4%) were male and 17 (58.6%) were female, with a mean age of 65.2 years. A majority of Whipple+ patients presented to the clinic with weight loss (58.6%), abdominal pain (55.2%), and/or jaundice (51.7%). When comparing Whipple+ to Whipple−, 31.0% of patients presented with nausea/vomiting compared to 17.8%, respectively (P=0.09). Twelve (41.4%) patients who underwent Whipple+ had a history of hypertension, compared to 234 (62.2%) patients who underwent Whipple− (P=0.03). A majority of Whipple+ patients (82.8%) had an ASA score of III, with only 2 (6.9%) having a score of IV.
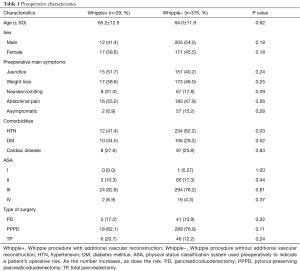
Full table
Perioperative characteristics
Perioperative characteristics of Whipple+ and Whipple− are presented in Table 2. The average operative time of Whipple+ and Whipple− was 553 (SD ±167) and 384 (SD ±139) min, respectively (P<0.0001). The average estimated blood loss of patients who underwent Whipple+ was 2,100 (SD ±3,869) mL compared to 362 (SD ±725) mL for patients who underwent Whipple− (P<0.0001).
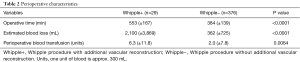
Full table
Postoperative characteristics
Postoperative characteristics including hospital stay, ICU stay, 30-day survival, 1-year survival, and 90-day readmission are presented in Table 3. Patients who underwent Whipple+ had a median [range] hospital and ICU stay of 12 [5–92] and 3 [0–59] days, respectively. Readmission rates of patients who underwent Whipple+ and those who underwent Whipple− was 31.0% and 14.6%, respectively (P=0.03). Survival rates were lower for patients who underwent Whipple+ compared to Whipple− patients. The 30-day survival rate for Whipple+ and Whipple− was 93.1% and 96.0%, respectively (P=0.35). The 1-year survival rates for the same groups was 55.2% and 83.5%, respectively (P<0.001).

Full table
Vascular reconstruction characteristics
One-year survival varied based on certain preoperative, perioperative, and postoperative characteristics. Ten (34.5%) patients had neo-adjuvant therapy with a 1-year survival rate of 40.0%, compared to 19 (65.5%) without neo-adjuvant therapy with a 1-year survival rate of 63.2%. In terms of the vessel resected/reconstructed and the type of reconstruction, a majority of the vascular reconstructions involved the PV (65.5%), with a 1-year survival rate of 52.6%, and were reconstructed via primary anastomosis (44.8%), with a 1-year survival rate of 53.8%. The 1-year survival of those who had interposition graft placement (27.6%) was 62.5%. The lowest survival rates were seen in reconstruction involving the PV (52.6%) and patch reconstruction (50.0%). Vascular surgeon assistance was utilized in 4 (13.8%) patients, with a 1-year survival rate of 75%. Overall, 4 (13.8%) patients were readmitted for complications involving the reconstruction. Of these 4, only 1 (3.4%) required re-intervention, and 2 (6.9%) required continued treatment for the complications and follow-up with vascular surgery.
Discussion
Preoperative discussion concerning treatment options and the associated postoperative outcomes are vital to any patient-orientated healthcare model. A study by Steffen et al. argued that engaging in preoperative discussion with patients focusing on treatment options and postoperative outcomes is crucial; especially for older patients undergoing high-risk procedures (25). This pertains directly to Whipple procedures involving vascular reconstruction, for the average patient is over 65 years of age (Table 1) and the surgery is associated with high mortality (Table 3).
Although a small number of patients undergoing a Whipple procedure for attempted resection of a pancreatic adenocarcinoma required additional major vascular resection and secondary reconstruction (7.2%), the results of the procedures reveal significant differences compared to those not requiring vascular reconstruction. The majority of these patients were female (58.6%) and had an ASA score of III (82.6%). Jaundice, weight loss, and abdominal pain were the most prevalent symptoms in both groups of patients, which is consistent with advanced pancreatic adenocarcinoma (2).
Compared to Whipple− procedures, Whipple+ procedures result in significantly increased operation time, estimated blood loss intraoperatively, and perioperative blood transfusion (Table 2). An increase in each of these characteristics of surgery are associated with increased odds of complications postoperatively (27,28). A study by Seykora et al. showed that increasing intraoperative blood loss during a PD was significantly correlated with poor perioperative outcomes, and that a main factor of increased blood loss was vascular resection (28). Therefore, this supports our findings that a Whipple+ procedure results in poor perioperative outcomes associated with increased operation time and intraoperative blood loss. This is supported by the results seen in Table 3, where 90-day readmission rate of Whipple+ procedures were significantly higher than that of Whipple− procedures. Nine (31.0%) of Whipple+ patients were readmitted within the first 90 days postoperatively, compared to 55 (14.6%) of Whipple− patients (P=0.03). Additionally, median ICU and hospital stay were both longer for Whipple+ patients compared to Whipple− patients (2 and 12 vs. 7 and 0, respectively). The 1-year survival rate of patients who underwent Whipple+ procedures was significantly less than those who received a Whipple− procedure (55.2% and 83.5%, respectively) (P<0.001). Interestingly, the 30-day survival was not significantly different between the two groups; however, the 30-day and 1-year survival rates were similar to those described previously in literature (Table 4). This may suggest that the vascular reconstruction has no significant short-term effect on patient outcome, but significantly affects the patient’s long-term recovery.
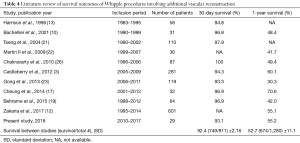
Full table
As for the characteristics of the vascular reconstruction, the majority of reconstructions involved the manipulation of the PV (65.5%), reconstruction via primary anastomosis (44.8%), no neo-adjuvant therapy (65.5%), no vascular surgeon assistance (86.2%), and negative margins of cancer (93.1%) (Table 5). Patients receiving neo-adjuvant therapy had a lower 1-year survival compared to those who were not (40.0% and 63.2%, respectively). In terms of the type of vessel resected and type of reconstruction, the 1-year survival was the lowest for PV resection (52.6%) and patch reconstruction (50.0%). The 1-year survival rates were highest for resection of the SMV (66.7%) and graft reconstruction (62.5%). Interestingly, Tee et al. further investigated the impact of the type of resection, arterial type, and use of neoadjuvant therapy in 111 patients who require a pancreatectomy with vascular reconstruction and found that none had a significant impact on long-term survival (29). Reconstructions performed by vascular surgeons (13.8%) had a 1-year survival of 75%. This suggests that vascular surgeon assistance during major vascular reconstruction may lead to a better chance of survival. Of the 29 patients who underwent a Whipple+ procedure, only 4 (13.8%) were readmitted due to complications associated with the reconstruction. Of these 4, 1 (3.4%) required re-intervention and 2 (6.9%) required continued follow-up with vascular surgery. An analysis of a larger number of Whipple+ patients would allow for a better understanding of the impact specific intra-operative characteristics have on patient outcomes.
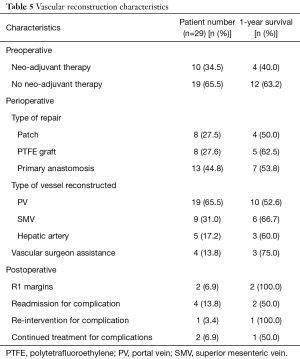
Full table
To this day, there is a debate regarding the feasibility and safety of the procedure. Many studies argue for feasibility of the procedure (10-22), while some, like this study, report data suggesting that vascular reconstruction during Whipple procedures result in significantly lower survival rates and increased complications (3,12,16,23,24). This present study utilizes the arguing studies’ results, along with the results of this study, to develop a clearer image of the postoperative outcomes associated with Whipple procedures involving vascular reconstruction. As seen in Table 4, a majority of studies report relatively similar 30-day survival rates (SD ±2.16%), while 1-year survival rates had more variation (SD ±11.1%). Of note, Cheung et al. reported 70.6% 1-year survival rate for patients receiving vascular reconstruction. The higher value is likely due to the group excluding patients with unresectable adenocarcinoma of the pancreas, who were deemed physically unfit for major PD, were considered to have long-segment arterial encasement of the tumors, or received only bypass graft operations, from analysis. In order to develop a better representation of expectant survival following Whipple procedure involving vascular reconstruction, the combination of the total number of patients with 30-day survival and 1-year survival in each study were divided by the total number of patients in the study. The 30-day and 1-year survival rates were 92.4% (749/811) and 52.7% (674/1,280), respectively (Table 4). Therefore, vascular reconstruction during Whipple procedures has just over 90% of 30-day survival, and just over 50% of 1-year survival. The present study increases the body of evidence in the field of Whipple procedures involving vascular resection with secondary reconstruction, while also analyzing previous and recent evidence in order to generate a more accurate postoperative expectation that can be relayed to the patient preoperatively.
Conclusions
Compared to conventional Whipple procedures, those requiring additional major vascular reconstruction are associated with decreased survival and increased readmission. When vascular reconstruction is a valid option, patients should be well aware of the associated outcomes prior to making their decision regarding treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was performed using data collected from a Mayo Clinic Institutional Review Board (IRB)-approved prospective database (IRB 09-00-3940). Informed consent was waived by the IRB as this study was deemed minimal risk to patients.
References
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Qiu M, Qiu H, Jin Y, et al. Pathologic Diagnosis of Pancreatic Adenocarcinoma in the United States: Its Status and Prognostic Value. J Cancer 2016;7:694-701. [Crossref] [PubMed]
- Castleberry AW, White R, De La Fuente SG, et al. The Impact of Vascular Resection on Early Postoperative Outcomes after Pancreaticoduodenectomy: An Analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Ann Surg Oncol 2012;19:4068-77. [Crossref] [PubMed]
- Glanemann M, Shi B, Lian F, et al. Surgical strategies for treatment of malignant pancreatic tumors: extended, standard, or local surgery? World J Surg Oncol 2008;6:123. [Crossref] [PubMed]
- Stojadinovic A, Hoos A. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg 2003;196:954-64. [Crossref] [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas- 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [Crossref] [PubMed]
- Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009;16:836-47. [Crossref] [PubMed]
- Saraee A, Vahedian-Ardakani J, Saraee E, et al. Whipple procedure: a review of a 7-year clinical experience in a referral center for hepatobiliary and pancreas diseases. World J Surg Oncol 2015;13:98. [Crossref] [PubMed]
- Warshaw AL, Thayer SP. Pancreaticoduodenectomy. J Gastrointest Oncol 2004;8.
- Bachellier P, Nakano H, Oussoultzoglous PD, et al. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwile? Am J Surg 2001;182:120-9. [Crossref] [PubMed]
- Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: persistent controversy and current recommendations. Ann Surg Oncol 2009;16:789-91. [Crossref] [PubMed]
- Zakaria HM, Stauffer JA, Harada E, et al. Portal and mesenteric vein resection during pancreaticoduodenceomy and total pancreatectomy. Egypt J Surg 2017;36:352-59. [Crossref]
- Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma: A contraindication for resection? Ann Surg 1996;224:342-7. [Crossref] [PubMed]
- Sasson AR, Hoffman JP, Ross EA, et al. En bloc resection for locally advanced cancer of the pancreas: is it worthwhile? J Gastrointest Surg 2002;6:147-57. [Crossref] [PubMed]
- Tucker ON, Rela M. Controversies in the management of borderline resectable proximal pancreatic adenocarcinoma with vascular involvement. HPB Surg 2008;2008:839503. [Crossref] [PubMed]
- Xie ZB, Gu JC, Zhang YF, et al. Portal vein resection and reconstruction with artificial blood vessels is safe and feasible for pancreatic ductal adenocarcinoma patients with portal vein involvement: Chinese center experience. Oncotarget 2017;8:77883-96. [Crossref] [PubMed]
- Cheung TT, Poon RTP, Chok KSH, et al. Pancreaticoduodenectomy with vascular reconstruction for adenocarcinoma of the pancreas with borderline resectibility. World J Gastroenterol 2014;20:17448-55. [Crossref] [PubMed]
- Ravikumar R, Sabin C, Abu Hilal M, et al. Impact of portal vin infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg 2017;104:1539-48. [Crossref] [PubMed]
- Beltrame V, Gruppo M, Pedrazzoli S, et al. Mesenteric- Portal Vein Resection during Pancreatectomy for Pancreatic Cancer. Gastroenterol Res Pract 2015;2015.
- Shimada K, Sano T, Sakamoto Y, et al. Clinical Implications of Combined Portal Vein Resection as a Palliative Procedure in Patients Undergoing Pancreaticoduodenectomy for Pancreatic Head Carcinoma. Ann Surg Oncol 2006;13:1569-78. [Crossref] [PubMed]
- Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy With Vascular Resection: Margin Status and Survival Duration. J Gastrointest Surg 2004;8:935-49. [Crossref] [PubMed]
- Martin RCG II, Scoggins CR, Egnatashvili V, et al. Arterial and Venous Resection for Pancreatic Adenocarcinoma. Arch Surg 2009;144:154-9. [Crossref] [PubMed]
- Gong Y, Zhang L, He T, et al. Pancreaticoduodenectomy combined with vascular resection and reconstruction for patients with locally advanced pancreatic cancer: a multicenter, retrospective analysis. PLoS One 2013;8:e70340. [Crossref] [PubMed]
- Kurosaki I, Hatakeyama K, Minagawa M, et al. Portal Vein Resection in Surgery for Cancer of Biliary Tract and Pancreas: Special Reference to the Relationship Between the Surgical Outcome and Site of Primary Tumor. J Gastrointest Surg 2008;12:907-18. [Crossref] [PubMed]
- Steffens NM, Tucholka JL, Nabozny MJ, et al. Engaging Patients, Providers and Community Members to Develop a Tool to Improve Preoperative Decision Making for Older Adults Facing High-Risk Surgery. JAMA Surg 2016;151:938-45. [Crossref] [PubMed]
- Chakravarty KD, Hsu JT, Liu KH, et al. Prognosis and feasibility of en-bloc vascular resection in stage II pancreatic adenocarcinoma. World J Gastroenterol 2010;16:997-1002. [Crossref] [PubMed]
- Jackson TD, Wannares JJ, Lancaster RT, et al. Does speed matter? The impact of operative time on outcome in laparoscopic surgery. Surg Endosc 2011;25:2288-95. [Crossref] [PubMed]
- Seykora TF, Ecker BL, McMillan MT, et al. The Beneficial Effects of Minimizing Blood Loss in Pancreatoduodenectomy. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Tee MC, Krajewski AC, Groeschl RT, et al. Indications and Perioperative Outcomes for Pancreatectomy with Arterial Resection. J Am Coll Surg 2018;227:255-69. [Crossref] [PubMed]


