Novel use of proton beam therapy for neoadjuvant treatment of radiation-associated squamous cell carcinoma of the esophagus
Introduction
Radiation-associated secondary solid neoplasms (SSN) are rare but significant complications, especially in long-term survivors receiving treatment at a young age, during prior eras with large treatment fields, or with concurrent chemotherapy (1-4). Modern radiation therapy (RT) techniques may render our current evidence regarding the risks and incidence obsolete; however, given the indolent course of SSN, as well as excellent survival rates achieved for pediatric and hematologic malignancies, we are often confronted with patients treated during historical decades with outdated techniques (1,4,5).
When encountering patients with an SSN, multidisciplinary evaluation is paramount given the morbidity associated with surgery or re-irradiation within a prior treatment field. This is especially true for SSN within the thorax, such as esophagus or lung, due to chemotherapy and radiation-related cardiopulmonary toxicity (6). RT coverage, and subsequent tumor control, is often compromised to maximally spare the heart, lungs, and spinal cord. Due to its intrinsic physical properties, proton beam therapy (PBT) facilitates adequate coverage of targets while achieving remarkably low dose to surrounding organs-at-risk (OAR). We believe these three cases of radiation-associated esophageal cancer are exemplary to highlight the benefit of protons over photons in this clinical circumstance. Informed consent for case presentation was obtained from each patient.
Case presentation
Patient A
A 46-year-old man was previously treated for stage III mediastinal diffuse large B-cell lymphoma with six cycles of R-CHOP and involved field RT to 36 Gy over 5 years prior to presentation. He had also completed multiple cycles of systemic therapy and stem cell transplant for recurrent/refractory disease over the ensuing 5 years. He developed treatment-related pulmonary fibrosis and cardiomyopathy. Surveillance CT incidentally showed thickening of the mid-esophagus. Endoscopy revealed a lesion 25 to 31 cm from the incisors, and biopsy confirmed squamous cell carcinoma (SCC). There were no regional or distant metastases on PET-CT. He was clinically staged as T2N0.
Patient B
A 62-year-old woman received mantle/para-aortic RT to 36 Gy for Hodgkin’s lymphoma (HL) 30 years prior to presentation. She developed coronary artery disease and aortic stenosis requiring an aortic valve replacement. After years of remaining HL-free, she developed progressive solid food dysphagia. CT revealed a 5.9-cm mid-esophageal mass, and endoscopy showed a circumferential lesion at 25 to 32 cm from the incisors; two enlarged para-esophageal lymph nodes were visualized. Biopsy of the primary mass revealed SCC. There was no evidence of distant metastases on PET-CT. She was clinically staged as T3N1.
Patient C
A 51-year-old woman was diagnosed with metastatic osteosarcoma at age 10, treated with multiple cycles of chemotherapy (including doxorubicin), left lung metastatectomy, and whole left lung RT to 15 Gy. By age 30, she developed cardiomyopathy and atrial/ventricular conductive abnormalities requiring cardiac pacemaker/defibrillator. She ultimately developed progressive solid food dysphagia. Endoscopy showed a 6-cm mass at 33 to 37 cm from the incisors; biopsy revealed SCC. There was no evidence of distant metastases on PET-CT. She was clinically staged as T3N0.
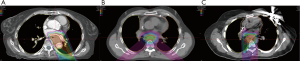
Treatment considerations (Figures 1-4)
Patient A
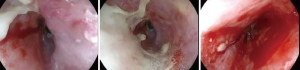
He was considered a high-risk operative candidate given treatment-related pulmonary fibrosis, and definitive RT with FOLFOX chemotherapy was pursued (7). Notably, there was a maximum point dose of 50 Gy immediately adjacent to the esophageal tumor from the prior RT course delivered 5 years prior. PBT was utilized with a passively scattered (PS) technique using a single left posterior oblique (LPO) field (Figure 1A). He experienced moderate/severe esophagitis with a single episode of hematemesis during the treatment course, as well as an esophageal stricture requiring balloon dilation (Figure 2). Though initially unplanned, he ultimately underwent a 3-hole minimally invasive esophagectomy (MIE) two months after the RT completion, which revealed moderate treatment response (ypT2) and seven negative lymph nodes. Surgery was complicated by intraoperative cardiac arrest and cardiogenic shock requiring four days of veno-arterial extracorporeal membrane oxygenation (ECMO) support. He recovered, and he remains disease free twenty-two months following surgery.
Patient B
Neoadjuvant RT with concurrent carboplatin/paclitaxel followed by esophagectomy was pursued (6). PBT was used with a PS technique using two posterior oblique fields (Figure 1B). The patient tolerated treatment well with only mild odynophagia that resolved 1 month post-RT. Two months after RT completion, the patient successfully underwent an Ivor-Lewis esophagectomy, which revealed a pathologic complete response with 13 negative lymph nodes. She remains disease free 6 years following surgery.
Patient C
She was considered a poor operative candidate due to treatment-related cardiac comorbidities, and definitive RT with FOLFOX chemotherapy was pursued (7). PBT with pencil-beam scanning (PBS) technique was used, employing a single posterior-anterior (PA) field at midline to avoid lung entrance due to prior whole lung RT (Figure 1C). She tolerated treatment well with only moderate odynophagia that resolved 1 month post-RT. Given excellent tolerance to therapy, she underwent an uncomplicated 3-hole MIE, which revealed residual SCC with treatment effect (ypT3) and 19 negative lymph nodes. She remains free of disease 26 months following surgery.
Protons versus photons
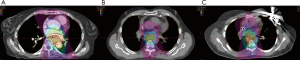
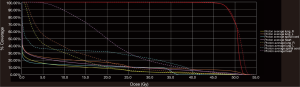
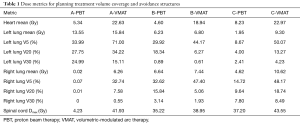
Each patient was treated with an initial course of 45 GyE [CTV expansion =3.5 cm superior/inferior, 1.5 cm circumferential; radiobiological equivalence (RBE) =1.1]; patient A and C received a primary tumor boost of 5.4 GyE. To demonstrate the benefit of protons over photons in this circumstance, a comparison plan using our institutional standard volumetric-modulated arc therapy (VMAT) was constructed for each patient. Each VMAT plan was designed to meet similar target volume coverage while maximally sparing the spinal cord, heart, and lungs. Specifically, two 360-degree coplanar arcs (clockwise/counterclockwise) were utilized. Representative VMAT plans are shown in Figure 3A,B,C. As demonstrated in Figure 4, PBT resulted in markedly decreased OAR metrics with equivalent target volume coverage. As seen in Table 1, however, V20Gy/V30Gy (i.e., volume of lung receiving 20 and 30 Gy) of lung was higher in patients A and B treated with PS technique.
Full table
Discussion
Each patient in this series developed a radiation-associated SCC of the thoracic esophagus. Given prior irradiation of the thorax +/− cardiopulmonary-toxic chemotherapy, these patients represent challenging cases since standard of care remains combined modality therapy for esophageal cancer (6). Patient A and C were considered suboptimal surgical candidates and initially treated with definitive RT and concurrent FOLFOX chemotherapy (8,9). Given favorable tolerance to chemoradiation, both ultimately underwent MIE, though patient A developed intraoperative cardiac arrest requiring significant hemodynamic support in setting of prior treatment-related pulmonary fibrosis and cardiomyopathy. While patient A and C had residual viable tumor at the time of resection, both remain free of disease recurrence approximately two or more years following treatment. Patient B, on the other hand, was the only patient with clinically positive lymph nodes and achieved a pathologic complete response with neoadjuvant PBT.
Currently there are no comparative studies evaluating the efficacy and safety of protons versus photons for esophageal cancer. To assess the potential benefit of PBT in these patients, a comparator VMAT plan was chosen per our institutional standard, given evidence that supports improved moderate-dose dosimetry and clinical toxicity compared to 3D-conformal RT and intensity modulated RT in esophageal cancer (10-12). PBT, especially with PBS technique, achieved markedly improved normal tissue avoidance while maintaining similar target volume coverage.
Technical considerations unique to PBT are important to consider. Beam range uncertainties due to inaccurate CT Hounsfield units-to-proton stopping power conversion remain a concern, and our practice is adding an extra 3.5% to the beam range to avoid potential undershooting. Secondly, there is concern for rise in the particle’s linear energy transfer at the end-of-range, which may lead to higher RBE at the distal margin of target. These effects are not currently included during plan optimization due to the lack of clinically accurate models. Given that the heart often lies immediately anterior to the esophagus, there is theoretical concern for a higher RBE dose being deposited to the heart (especially left atrium). However, with a remarkably low cardiac dose is achieved with PBT that is deposited away from the ventricles and left anterior descending artery, the implications of this dosimetry may be of little clinical relevance.
In this series, patient A and B were treated with PS protons, while patient C was treated after routine implementation of PBS. There are potential drawbacks with the PS technique utilizing cord-avoiding PO fields entering through the lung (patient A and B). As seen in patient A, the left lung high-dose exposure was greater with a single LPO field due to larger modulation required to cover the target anteriorly. Similarly, for patient B, there is more high-dose exposure in both lungs due to utilization of two PO fields, compared to a VMAT plan that allows additional dose modulation through the spine and heart/mediastinum. For patient B, the use of two PO fields was utilized to mitigate beam range uncertainty and concern of increased linear energy transfer (LET, i.e., dose deposition) into the heart/aortic valve considering this patient’s cardiac and valvular disease from prior RT. Nonetheless, the increased high-dose lung exposure is the expense of reduced high-dose regions in the heart/mediastinum and spinal cord. Furthermore, patient B successfully underwent an esophagectomy without acute pulmonary complication and remains disease-free without clinical or radiographic pulmonary toxicity 6 years after treatment. For patients with more significant pulmonary comorbidities, PBS approach with a single PA field appears optimal, like that utilized for patient C, which allows for minimal high-dose delivery to lung while maintaining low dose to spinal cord due to superior modulation compared to PS. Additionally, minimizing the spot size (sigma) of the pencil-beam is crucial to narrowing the distribution of dose at the end-of-range and thereby minimizing the amount of distal OAR at risk of higher dose deposition.
Conclusions
This case series demonstrates a clinical use, as well as safety and efficacy, of PBT for esophageal cancer. Specifically, this advanced technique may be suitable for patients with prior thoracic RT and/or cardiopulmonary-toxic chemotherapy. While the clinical outcomes for these patients are encouraging, the routine utilization of protons for neoadjuvant or definitive chemoradiation should be prospectively compared against conventional photon modalities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys 2013;86:224-33. [Crossref] [PubMed]
- Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood 2002;100:1989-96. [Crossref] [PubMed]
- De Bruin ML, Sparidans J, van't Veer MB, et al. Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 2009;27:4239-46. [Crossref] [PubMed]
- Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 2007;25:1489-97. [Crossref] [PubMed]
- Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083-95. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 2014;15:305-14. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus 2015;28:352-7. [Crossref] [PubMed]
- Zhang WZ, Zhai TT, Lu JY, et al. Volumetric modulated arc therapy vs. c-IMRT for the treatment of upper thoracic esophageal cancer. PLoS One 2015;10:e0121385. [Crossref] [PubMed]
- Yang H, Feng C, Cai BN, et al. Comparison of three-dimensional conformal radiation therapy, intensity-modulated radiation therapy, and volumetric-modulated arc therapy in the treatment of cervical esophageal carcinoma. Dis Esophagus 2017;30:1-8. [PubMed]