The accuracy of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as a marker for gastrointestinal malignancies
Introduction
Gastrointestinal (GI) cancers remain a significant cause of cancer related mortality (1) with those patients who present with node positive or metastatic diseases exhibiting worse overall survival (2). Recurrences remain high and significantly impact outcomes with 5-year survivals less than 10% (3). It has been well established that early detection of GI malignancies could potentially result in an overall reduction in cancer related mortality via earlier interventions (4,5). Additionally, prognostic tools that could more accurately stage GI tumors could better guide therapeutic interventions (6).
The current prognosis of most GI cancers is dependent upon the TNM staging system. Unfortunately, simple accurate predictors for the presence of node positivity and metastatic disease are lacking. The current methods of assessing node positivity, metastatic and recurrent disease relies on laparoscopy, endoscopy, surgical resection, CT imaging or PET scanning (7). These methods may lead to increased time between identification of disease extent and initiation of the next stage of treatment. Unfortunately there are no simple clinical methods for predicting the likelihood of nodal positivity, recurrence, or metastatic disease (8).
Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) have been investigated as potential markers in a number of cancers ranging from breast to GI malignancies; when there is an increased NLR or PLR, the cancer specific survival for patients decreases (3,9-14). Additionally, it has been suggested that combining NLR and PLR may further enhance the ability for predicting prognosis than using each independently (15). Some authors have correlated NLR and PLR with lymph node positivity, but there is little data utilizing these markers for predicting lymph node positivity among gastrointestinal cancers (16,17). A high NLR value has also been implicated as predictive for a patient to develop a recurrence in lung, hepatocellular, esophageal, and oral-pharyngeal malignancies (18-21). However, the predictive capacity of NLR and PLR in assessing risk of recurrence after surgical resection of GI malignancies remains unknown.
We sought to examine the individual and combined predictive potential of NLR and PLR in evaluating lymph node positivity, metastatic and recurrent disease in patients with gastrointestinal malignancies.
Methods
This study was approved by the institutional review board at Sarasota Memorial Institute for Cancer Care. A prospectively maintained gastrointestinal database was queried to identify patients with who underwent treatment between 2014–2016. The malignancies included in this study were: esophageal, pancreatic, colorectal, gastric, and biliary. NLR and PLR values were determined via complete blood counts (CBC) that were taken throughout treatment. The NLR was defined as the neutrophil count divided by the total lymphocyte count. PLR was determined as the absolute platelet count divided by the total lymphocyte count. The baseline value for determining an elevated PLR or NLR value was determined from previously published studies on PLR or NLR (18-21). The associations between PLR and NLR and outcomes were compared using Chi-squared or Fisher’s exact tests as appropriate. Each of these tests was two-sided and a P value of <0.05 was considered statistically significant.
Predicting lymph node positivity and metastases
Each patient was analyzed based on their PLR and NLR values before and after neoadjuvant therapy (NT). In those that did not undergo NT, the NLR and PLR values were obtained via a CBC taken immediately before surgery of the primary tumor. Lymph node positivity and metastatic disease were determined via surgical resection and post-operative pathological staging.
Predicting recurrence
Patients that had metastatic disease identified before surgery were discounted from the recurrence analysis. CBC values were taken pre-surgical intervention and 2 weeks post-surgery. Normal follow-up after surgery included a 3-month visit to determine baseline normalization of NLR and PLR. Recurrence of disease was identified via CT scans, PET scans, endoscopy, or laparoscopy and all were confirmed via biopsy. Once pathologic recurrences were confirmed, NLR and PLR values recorded after patients completed all interventions were compared with new NLR and PLR values.
Results
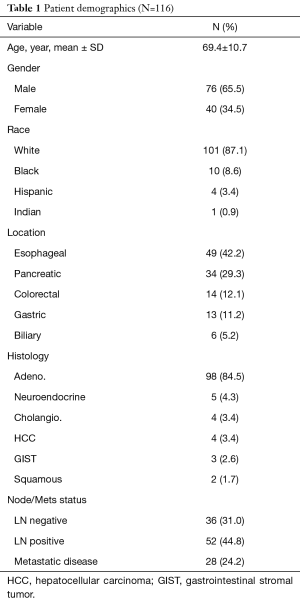
We identified 116 patients who were diagnosed with gastrointestinal malignancies, 76 (65.5%) were male and 40 (34.5%) were female with an average age of 69.4±10.7 years (Table 1). The mean follow up was 14.1±15.5 months. Of those, 49 (42.2%) were esophageal cancer, 34 (29.3%) pancreatic cancer, 14 (12.1%) colorectal cancer, 13 (11.2%) gastric cancer, and 6 (5.2%) biliary cancers. Among all the patients, 36 (31.0%) had node negative disease, 52 (44.8%) had node positive disease, and 28 (24.2%) patients had metastatic disease preoperatively. Those patients with metastatic disease, 4 (3.4%) were found during staging laparoscopy, and 24 (20.6%). were diagnosed preoperatively by imaging.
Full table
Predicting LN+ disease and metastases
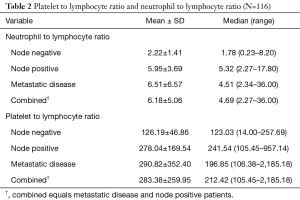
Lymph node (LN) negative patients exhibited a median and mean NLR value of 1.78 (0.23–8.20) and 2.22±1.41 respectively. Patients with lymph node positivity or metastatic disease exhibited a higher median and mean NLR of 4.69 (2.27 to 36) and 6.18±5.06 compared to LN− patients, P<0.001. There were minimal differences between the NLR for the node positive and metastatic disease patients. The patients with metastatic disease had slightly higher mean NLR 6.51±6.57 compared to the patients determined to have positive lymph node involvement 5.95±3.69, P=0.63.
PLR values for patients with LN− disease exhibited a median and mean value of 123.03 (14–257.69) and 126.19±46.86 respectively (Table 2). In lymph node positive and metastatic disease patients the median and mean PLR were higher compared to LN− patients 212.42 (105.45–2,185.18) and 283.38±259.95 respectively, P<0.001. Similar to NLR values, the mean PLR was slightly increased in the metastatic patients compared to the LN+ patients 290.82±352.40 and 278.04±169.54 respectively, P=0.83.
Full table
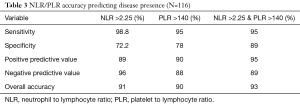
Utilizing NLR and PLR cut off numbers of 2.25 and 140 the sensitivity, specificity, positive predictive value, and negative predictive value and overall accuracy were determined. The sensitivity for NLR (98.8%) was higher compared to that of PLR (95%) with a combined sensitivity of NLR and PLR of 95%. Conversely, the specificity for PLR (78%) was greater than that of NLR (72.2%). The combined specificity for PLR and NLR was 89%. The positive predictive value (PPV) is comparable for both PLR (90%) and NLR (89%), while the combined PPV is greater than both at 95%. The negative predictive value (NPV) was much greater for NLR (96%) than the PLR (88%), but the combined NPV was 89%. The overall accuracy for NLR was 91%, for PLR 90%, and the combined accuracy of both the NLR and PLR was 93% (Table 3).
Full table
Predicting recurrence
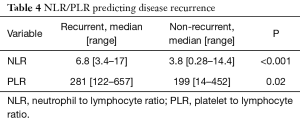
There were 23 (19.8%) patients identified as having metastatic disease who did not undergo surgery that were excluded from recurrence analysis. Recurrences were identified in 27 (29%) patients after definitive treatment with a median time to recurrence of 6 [3–26] months. Sites of systemic recurrences included: liver 12 (44.4%), lung 5 (18.5%), peritoneal 5 (18.5%), nodal 4 (14.8%), and bone 1 (3.8%). Of these, patients with LN positive disease 18 (34.6%) at their original treatment were more likely to develop recurrence then those with negative lymph nodes 9 (25%), P=0.04. The median NLR for patients with recurrence was 6.8 (3.4–17) and the non-recurrent patients had a lower median NLR at 3.8 (0.28–14.4), P<0.001. The median PLR was 281 [122–657] for recurrences and 199 [14–452] for non-recurrent patients P=0.02 (Table 4).
Full table
Discussion
Currently, tumor markers are inaccurate in their ability to predict specific GI cancers in a cheap and effective way. A number of markers have been previously studied including carcinoembryonic antigen (CEA) and CA19. The monitoring of CEA in colorectal cancers provided only a moderate increase in patient outcomes due to poor sensitivity and specificity of the CEA assay (22). CA19 was shown to be effective in pancreatic cancer detection with specificity and sensitivities around 80%, which leaves a significant number of recurrences undiagnosed (23). Thus, the need for additional more accurate markers such as NLR and PLR are paramount to the improvement in diagnosis of locally advanced/metastatic and recurrence disease.
We have demonstrated that neutrophil-lymphocyte and platelet-lymphocyte ratios are significant predictors of lymph node positivity/metastatic disease and disease recurrence in gastrointestinal malignancies. Although an elevated NLR or PLR has already been extensively linked to decreased survival (12-19), this is the first study to correlate NLR and PLR to lymph node positivity and metastatic disease. While both NLR and PLR have been investigated individually, (15,20), Feng et al. determined that a combination of PLR and NLR had a superior predictive value than individual PLR or NLR for esophageal squamous cell carcinoma (24). We corroborated these results in our own esophageal patients and demonstrated their prognostic potential and overall improvement in the accuracy in other gastrointestinal malignancies.
NLR and PLR have been investigated in non-gastrointestinal cancers, and found to correlate to increased recurrence rates (19-21). An elevation in these values in hepatocellular and pancreatic cancer similarly showed that PLR was predictive for cancer recurrence (20,25). Our data shows that both PLR and NLR are predictive of cancer recurrence. While an elevated PLR and NLR has been associated with poorer prognosis this mechanism is poorly understood. Some authors have suggested this to be a function of a larger primary tumor (26), we did not find this to be case as elevation of NLR and PLR were indicative of LN positivity and metastatic disease irrespective of size of primary tumor.
The presence of a systemic immune response in cancer patients is well recorded and numerous inflammatory cells have been found in tumors. Elevation of NLR and PLR may be due to decreased lymphocyte proliferation, which can enhance tumorigenesis and spread from the reduction of antitumor mechanisms (27). It has also been recorded that in sites of metastasis, there is a large number of leukocytes present, which may promote the growth and progression of the cancer (28,29). Cancer growth and lymph node metastasis have also been linked to an increased local immune status (30).
Thrombocytosis has been shown to decrease survival in patients diagnosed with lung cancer (31). Megakaryocytes are induced by cytokines such as IL-1 and IL-2 resulting in thrombocytosis (32). This systemic inflammatory response to the tumor leads to an increase in platelet counts. The associated increases of PLR and NLR could be attributed to this local immune condition created by the tumor. Thus, the increased number of neutrophils in the NLR is likely due the high density of neutrophils in the metastases. Malignancies also have the ability to activate anergic tolerance by lymphocytes or by expressing a suppressive influence on immune response through various cytokines (33,34). The depression of the innate immune response common in cancers is best seen by the reduction in the helper T-cell lymphocytes (35). Many of the patients in our study underwent chemotherapy prior to surgical resection of the cancer. It has been well documented that chemotherapy decreases lymphocyte numbers and overall immune function (36).
As of now, there are no definitive baseline values that constitute an elevated NLR or PLR that will indicate an increase in tumor burden. Some authors have suggested that a NLR [2–4] and PLR [120–200] indicates the presence of locally advanced or metastatic disease (15,37-39). There is likely to be no definitive baseline value that can be confirmed due to genetic variations in lymphocyte numbers and antigen affinities that differ within populations. Additionally, while the NLR and PLR can predict lymph node involvement or metastatic disease, it cannot be used to differentiate between the two. Therefore, these values should be used in conjunct with imaging to further identify extent of tumor burden.
Conclusions
We have demonstrated that neutrophil-lymphocyte and platelet-lymphocyte ratios are significant predictors of lymph node positivity and metastatic disease in patients diagnosed with gastrointestinal malignancies. Combining the predictive capabilities of NLR and PLR resulted in an accuracy of over 90% which was significantly improved over their individual accuracies. Additionally, NLR and PLR were able to diagnose recurrent disease and thus can be pivotal for surveillance in patients undergoing curative resection. Failure of normalization of NLR and PLR after surgery may be a marker for persistent disease or early metastatic disease unable to be identified by current imaging techniques mandating closer follow-up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board at Sarasota Memorial Institute for Cancer Care.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Mansfield CM, Kramer S, Southard ME, et al. Prognosis in patients with metastatic liver disease diagnosed by liver scan. Radiology 1969;93:77-84. [Crossref] [PubMed]
- Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 2013;109:395-400. [Crossref] [PubMed]
- Watanabe M, Baba Y, Nagai Y, et al. Minimally invasive esophagectomy for esophageal cancer: an updated review. Surg Today 2013;43:237-44. [Crossref] [PubMed]
- Hopper AD, Campbell JA. Early diagnosis of oesophageal cancer improves outcomes. Practitioner 2016;260:23-8, 3.
- Liu X, Long Z, Cai H, et al. Analysis of lymph node metastasis correlation with prognosis in patients with T2 gastric cancer. PLoS One 2014;9. [Crossref] [PubMed]
- Rau B, Hünerbein M, Reingruber B, et al. Laparoscopic lymph node assessment in pretherapeutic staging of gastric and esophageal cancer. Recent Results Cancer Res 1996;142:209-15. [Crossref] [PubMed]
- Tavares A, Viveiros F, Maciel J, et al. Conventional clinical and pathological features fail to accurately predict recurrence in patients with gastric cancer staged N0. Eur J Gastroenterol Hepatol 2015;27:425-9. [Crossref] [PubMed]
- Krenn-Pilko S, Langsenlehner U, Stojakovic T, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol 2016;37:361-8. [Crossref] [PubMed]
- Cheng H, Long F, Jaiswar M, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep 2015;5:11026. [Crossref] [PubMed]
- Ishizuka M, Oyama Y, Abe A, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol 2014;110:935-41. [Crossref] [PubMed]
- Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. [Crossref] [PubMed]
- Jung J, Park SY, Park SJ, et al. Prognostic value of the neutrophil-to-lymphocyte ratio for overall and disease-free survival in patients with surgically treated esophageal squamous cell carcinoma. Tumour Biol 2016;37:7149-54. [Crossref] [PubMed]
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. [Crossref] [PubMed]
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [Crossref] [PubMed]
- Pang W, Lou N, Jin C, et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol 2016;28:493-502. [Crossref] [PubMed]
- Khan AA, Akritidis G, Pring T, et al. The Neutrophil-to-Lymphocyte Ratio as a Marker of Lymph Node Status in Patients with Rectal Cancer. Oncology 2016;91:69-77. [Crossref] [PubMed]
- Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer. Clin Transl Oncol 2015;17:772-8. [Crossref] [PubMed]
- Ozturk K, Akyildiz NS, Uslu M, et al. The effect of preoperative neutrophil, platelet and lymphocyte counts on local recurrence and survival in early-stage tongue cancer. Eur Arch Otorhinolaryngol 2016;273:4425-9. [Crossref] [PubMed]
- Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41. [Crossref] [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. [Crossref] [PubMed]
- Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem 2001;47:624-30. [PubMed]
- Gui JC, Yan WL, Liu XD. CA19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: a meta-analysis. Clin Exp Med 2014;14:225-33. [Crossref] [PubMed]
- Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 2013;6:1605-12. [PubMed]
- Garcea G, Ladwa N, Neal CP, et al. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg 2011;35:868-72. [Crossref] [PubMed]
- Chiang SF, Hung HY, Tang R, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis 2012;27:1347-57. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Koizumi K, Hojo S, Akashi T, et al. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci 2007;98:1652-8. [Crossref] [PubMed]
- Maehara Y, Tomisaki S, Oda S, et al. Lymph node metastasis and relation to tumor growth potential and local immune response in advanced gastric cancer. Int J Cancer 1997;74:224-8. [Crossref] [PubMed]
- Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J 1996;9:1826-30. [Crossref] [PubMed]
- Alexandrakis MG, Passam FH, Moschandrea IA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol 2003;26:135-40. [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10:909-15. [Crossref] [PubMed]
- Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 2007;18:171-82. [Crossref] [PubMed]
- Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med 1999;27:733-40. [Crossref] [PubMed]
- Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994;84:2221-8. [PubMed]
- Haruki K, Shiba H, Horiuchi T, et al. Neutrophil to Lymphocyte Ratio Predicts Therapeutic Outcome After Pancreaticoduodenectomy for Carcinoma of the Ampulla of Vater. Anticancer Res 2016;36:403-8. [PubMed]
- Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014;31:305. [Crossref] [PubMed]
- Namikawa T, Munekage E, Munekage M, et al. Evaluation of Systemic Inflammatory Response Biomarkers in Patients Receiving Chemotherapy for Unresectable and Recurrent Advanced Gastric Cancer. Oncology 2016;90:321-6. [Crossref] [PubMed]


