
An unusual case of obstructive jaundice: ampullary Burkitt lymphoma
Introduction
Primary lymphomas of the digestive tract are an uncommon heterogeneous group of neoplasms that occur primarily in the stomach. Primary gastric lymphoma constitutes 1.48% of all gastric cancers in children (1,2). Because of the paucity of lymphoid tissue in the duodenum, primary duodenal involvement of the lymphoma is a rare condition. It accounts for less than 5% of all small bowel lymphomas (3). Periampullary lymphoma of ampulla of Vater is a rare entity (4). We here report the case of a 14-year-old male who initially presented as cholestatic jaundice and was ultimately diagnosed as a case of primary ampullary Burkitt’s lymphoma. This case highlights the complexity and challenge in diagnosing and managing patients of primary lymphoma of ampulla of Vater which is a rare clinical entity with less than 5 cases reported in the literature (4-6) .We could not find any case reported of primary Burkitt’s lymphoma of ampulla of Vater in English literature.
Case presentation
A 14-year-old boy presented with history of jaundice for1 month, which was insidious in onset and gradually progressive without any preceding history of prodromal symptoms. It was associated with generalized pruritus without clay color stools. There was associated history of significant loss of appetite and loss of weight of around 15 kg in last 1 month. There was no history of fever, night sweats, abdominal pain, abdominal distension, abdominal lump, melena, hematemesis, or altered sensorium. He had no history of oral ulcers, arthralgia, joint pain, facial rash, neuropsychiatric manifestations, easy fatigability. There was no history of blood transfusions, major surgical illness or family history of jaundice or chronic liver disease. He was first born child of a non-consanguineous marriage. Patient did not take alternative medicines for jaundice.
On examination, he was hemodynamically stable with blood pressure of 110/70 mmHg, heart rate of 90/minute with BMI of 19 kg/m2. He had icterus but no pallor, peripheral lymphadenopathy or clubbing. There was presence of scratch marks on body and shiny nails. On abdominal examination, he was found to have hepatomegaly of 4 cm below right subcostal margin in midclavicular line, splenomegaly of about 4 cm below the left subcostal margin along the splenic axis and gall bladder was not palpable. Rest of his systemic examination was within normal limits.
As he presented with features of cholestatic jaundice the differential diagnosis initially considered in this case were progressive familial intrahepatic cholestasis, autoimmune hepatitis- overlap syndromes, Wilsons disease. In view of significant loss of weight and appetite causes of extrahepatic biliary obstructions like primary sclerosing cholangitis, choledochal cyst, choledocholithiasis, etc. were also considered in this patient.
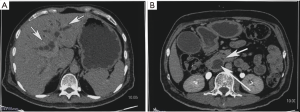
His routine blood investigations are described in Table 1. He had direct hyperbilirubinemia with raised alkaline phosphatase to 441 IU/L (normal <310 IU/L), raised serum gamma glutamyl transferases levels to 110 IU/L (normal <30 IU/L) and raised Alanine transaminases and Aspartate transaminases (6 times the upper normal limit). His viral markers including HbsAg, Ant-HCV, IgM anti-HAV, IgM anti-HEV were negative. Autoimmune markers including ANA, ASMA, AntiLKM-1 were negative. Serum Immunoglobulin G levels were normal. He subsequently underwent ultrasonography of abdomen which revealed hepatomegaly of 15.6 cm with moderate central and peripheral IHBRD and proximally dilated CBD. Contrast enhanced Computed tomography of abdomen revealed double duct sign with dilated common bile duct and pancreatic duct with no evidence of any mass lesion in distal CBD or pancreatic head suggestive of ampullary obstruction (Figure 1A,B) There was associated peripancreatic subcentimetric lymphadenopathy. His CA 19-9 was 709 mg/L.
Full table
Subsequently he underwent upper gastrointestinal (GI) endoscopy and side viewing endoscopy (Figure 2), which showed normal esophagus and stomach with a friable non-lumen occluding periampullary ulceroproliferative mass lesion of size approximately 2×2.5 cm2 distorting the papilla. Patient underwent Endoscopic retrograde cholangiopancreatography with selective cannulation of CBD which revealed diffusely dilated CBD throughout with distal CBD narrowing. A 10 Fr ×10 cm plastic biliary stent was placed with free flow of bile. Biopsy from the growth was taken.

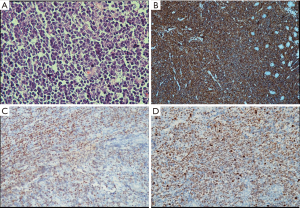
Histopathology and immunohistochemistry report from the mass lesion was suggestive of high grade non-Hodgkin’s lymphoma B-phenotypic positive for CD 20, BCL 6 and negative for BCL 2 with MIB-1 index of >95% (Figure 3A,B,C,D). Diagnosis of extranodal Burkitt’s lymphoma was made following which he underwent CT thorax which was normal and bone marrow examination which showed reactive marrow with no evidence of neoplastic cells. He had normal CT brain study. CSF cytology was negative for malignant cells. PET-CT done which revealed metabolically active ill-defined soft tissue mass in the D2 region of duodenum with no evidence of any other lesion.
He was started on R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, prednisolone) chemotherapy regimen which included 6 cycles of chemotherapy at an interval of 21 days. He responded to treatment well with gradual normalization of his symptoms and liver function tests. By the third cycle his symptoms dramatically improved and biliary stent was removed after the obstruction was relieved. Imaging with CT scan and side viewing endoscopy revealed significant reduction in size of the mass lesion. He is currently under regular follow up.
Discussion
Primary extranodal lymphomas are heterogeneous group of diseases having diverse etiopathogenesis, presentation and outcomes. Although lymphomas are the third most common tumor in children (5), primary non-Hodgkin lymphoma of the duodenum is an uncommon primary tumor of the GI tract representing only 5% to 16% of all small intestinal lymphomas (6,7) lymphomatous involvement of ampulla of Vater is further rare. Only few cases of primary ampullary lymphoma and no cases of primary ampullary Burkitt’s lymphoma are reported in the literature. It has a peak frequency at 7 years of age while it is rare before 2 years. It is more common among boys than among girls with a ratio of 3:1 (8,9). Diffuse large cell lymphoma of B-cell origin (Burkitt’s lymphoma) is currently recognized as representing the predominant histological type in these patients. Certain risk factors have been implicated in the pathogenesis of GI lymphoma including Campylobacter jejuni, Helicobacter pylori infection, Epstein-Barr virus, hepatitis B virus, human immunodeficiency virus, human T-cell lymphotropic virus-1, celiac disease, inflammatory bowel disease and immunosuppression (10,11). None of these were present in our patient. World health organization classifies Burkitt lymphoma as sporadic, endemic (African) and immunodeficiency associated.
The clinical presentation of small intestinal lymphoma is non-specific and patients have varied symptoms ranging from non-specific abdominal pain, nausea, vomiting, weight loss and rarely acute obstructive symptoms, intussusceptions, perforation or diarrhea. Obstructive jaundice is one of the rare presentations of small intestinal lymphoma. The macroscopic appearance of small intestinal lymphoma may range from a mass, polyp to an ulcer on endoscopy, which cannot be distinguished from other lesions. In our case the patient presented with cholestatic jaundice with loss of weight and side viewing endoscopy revealed an ulcerated mass lesion at the ampulla.
Microscopic examination as seen in our case usually reveals diffuse growth pattern of lymphoid cells with pleomorphism.
An important aspect to be considered is the increasing sensitivity and specificity of imaging techniques like endoscopic ultrasound (EUS) and PET-CT in the diagnosis of lymphomas. Ampullary carcinoma will have distal nodular mass at distal pancreatobiliary region on imaging and less commonly will show a mass at endoscopy while ampullary adenoma will have soft tissue mass with irregular ampullary margins often >1 cm and seen easily at endoscopy. The other differential could be distal cholangiocarcinoma which can easily be diagnosed on imaging which shows biliary dilatation with abrupt narrowing at the level of mass, intraductal polypoidal mass, wall thickening of the duct and delayed enhancement. The other differentials which should be considered and can be ruled out with the help of clinical, demographic and imaging features are duodenal cancer, periampullary lipoma, pancreatic adenocarcinoma, benign papillary stenosis. EUS has gained momentum as an integral tool in the diagnosis, locoregional staging, and monitoring response of GI lymphoma to treatment (12). PET scan was done in our case instead of EUS as surgical options were not considered in our case. The value of EUS and CT, however, is a matter of debate in the follow-up of patients. It is well established that histological remission precedes the normalization of wall changes in patients with lymphoma (12,13), and so imaging alone may not be sensitive enough to diagnose remission. Unlike adults, treatment in children and adolescent population is based on chemotherapy alone in most of the cases. Global overall 5-year survival rate combining all stages is 75%. Tumor regression may be spectacular in few days after starting chemotherapy. In our patient, symptoms were resolved dramatically by the third cycle of chemotherapy. Imaging after 3rd cycle revealed significant reduction in size of mass lesion and subsequently his biliary stent was removed after the obstruction was relieved. This highlights the need for early diagnosis of these rare tumors causing obstruction at periampullary region in preventing septic complications of cholangitis that may arise if obstruction persists. Correct histopathological diagnosis was key in preventing surgery in this patient.
Conclusions
Burkitt’s lymphoma of ampulla of Vater is a rare variety of extranodal Burkitt’s lymphoma and it is not easy to differentiate it from other tumors at this site clinically and radiologically. Endoscopic duodenal biopsy and accurate immunohistochemistry is essential diagnostic tool to correctly identify these rare tumors and plan optimal treatment strategy. With appropriate chemotherapy the clinical outcome may be excellent even when it presents as locally advanced tumors thus sparing the patient from undergoing a radical surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient and his father.
References
- Chieng JH, Garrett J, Ding SL, et al. Clinical presentation and endoscopic features of primary gastric Burkitt lymphoma in childhood, presenting as a protein-losing enteropathy: a case report. J Med Case Rep 2009;3:7256. [Crossref] [PubMed]
- Kesik V, Safali M, Citak EC, et al. Primary gastric Burkitt lymphoma: a rare cause of intraabdominal mass in childhood. Pediatr Surg Int 2010;26:927-9. [Crossref] [PubMed]
- Mundasad B, Hawe MJG. Lymphoma presenting as a bleeding duodenal ulcer: a case report. Internet J Trop Med 2005;3:abstr 1.
- Yildirim N, Oksüzoğlu B, Budakoğlu B, et al. Primary duodenal diffuse large cell non-hodgkin lymphoma with involvement of ampulla of Vater: report of 3 cases. Hematology 2005;10:371-4. [Crossref] [PubMed]
- Rahman S, Reyes E, Mehta A, et al. Primary Duodenal Lymphoma presenting as Obstructive Jaundice. Int J Gastroenterol 2008;7. Available online: http://ispub.com/IJGE/7/2/13052
- Tutara NU, Ozgula E, Avc Z, et al. An unsual cause of obstructive jaundice; computed tomography and ultrasound findings of duodenal non-Hodgkin’s lymphoma. Eur J Radiol Extra 2007;63:69-73. [Crossref]
- Ravindra KV, Stringer MD, Prasad KR, et al. Non-Hodgkin lymphoma presenting with obstructive jaundice. Br J Surg 2003;90:845-9. [Crossref] [PubMed]
- Pietsch JB, Shankar S, Ford C, et al. Obstructive jaundice secondary to lymphoma in childhood. J Pediatr Surg 2001;36:1792-5. [Crossref] [PubMed]
- Lupescu IG, Grasu M, Goldis G, et al. Computer tomographic evaluation of digestive tract non-Hodgkin lymphomas. J Gastrointestin Liver Dis 2007;16:315-9. [PubMed]
- Engels EA. Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:401-4. [Crossref] [PubMed]
- Müller AM, Ihorst G, Mertelsmann R, et al. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 2005;84:1-12. [Crossref] [PubMed]
- Di Raimondo F, Caruso L, Bonanno G, et al. Is endoscopic ultrasound clinically useful for follow-up of gastric lymphoma? Ann Oncol 2007;18:351-6. [Crossref] [PubMed]
- Boot H. Diagnosis and staging in gastrointestinal lymphoma. Best Pract Res Clin Gastroenterol 2010;24:3-12. [Crossref] [PubMed]


