
Exosomal markers (CD63 and CD9) expression and their prognostic significance using immunohistochemistry in patients with pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of death from cancer in men and women in the United States. A total of 55,440 new cases and 44,330 deaths are estimated to occur in 2018 (1). The prognosis of PDAC is notoriously poor, with a 5-year overall survival (OS) after pancreaticoduodenectomy is approximately 10% for node-positive disease while it is approximately 30% for node-negative disease (2). Tumor stage is the most important prognostic factor. Other prognostic factors for resectable PDAC are the status of surgical margins, tumor differentiation, presence of lymphatic invasion and CA 19-9 level (3).
A number of molecules have been explored as potential prognostic marker for patients with PDAC (4,5). In a systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with PDAC, none of the molecular markers described could be recommended for routine clinical use. They were identified in small cohorts and there were inconsistencies between studies (4). Genome-scale analysis in patients with early stage PDAC after pancreaticoduodenectomy provided a new prospect for prognostic biomarkers that might have clinical value in the future. Due to the limitations in the study, the findings still needed to be verified in large cohort studies prior to clinical application (6).
Exosomes are membranous nanovesicles [extracellular vesicles (EVs) of 30–150 nm diameter] of endocytic origin secreted by most cell types from diverse organisms (7). Depending on the cell of origin and the conditions for secretions, exosomes appear to contribute to a diverse range of biological processes and play pivotal role in mediating intercellular and distant communication by transferring various functional biomolecules including RNAs, DNA, lipids and proteins (7,8).
PDAC-derived exosomes are involved in pre-metastatic niche formation, distant metastasis and pro-metastatic microenvironment (9). In addition to their role in promoting metastasis, PDAC-derived exosomes facilitate epithelial-mesenchymal transition, enhance tumor cell invasiveness, promote vascular destruction and invasion and transport oncogenic abilities between different cell types (10). Moreover, PDAC-derived exosomes play novel roles in mediating acquired chemoresistance and immunomodulatory effects (11,12).
Exosomal membranes are enriched in endosome-specific tetraspanins (CD9, CD63, CD81) (12). In patients with PDAC, the expression of the exosomal markers (CD63 and CD9), using immunohistochemistry (IHC), is higher in malignant cells compared to adjacent normal cells (13,14). In patients with PDAC, positive correlation between CD9 expression and OS was reported (15). However, CD63 expression was conserved in all patients without reported prognostic significance. According to previously published reports, CD9 and CD63 are the most specific markers for exosomes (16,17). Therefore, CD9 and CD63 were selected for this study to explore the prognostic significance of CD63 and CD9 expression using IHC in patients with PDAC of mixed racial backgrounds.
Methods
Pancreatic tissue collection
This is a retrospective study conducted at the University of South Alabama-Mitchell Cancer Institute in Mobile, Alabama. Patients were identified through searching the pancreatic cancer database. The University of South Alabama Institutional Review Board (IRB) approved this study and the IRB-approved database provided a waiver of the requirement for informed consent and allowed for publication of de-identified data.
Immunohistochemical analysis
Two 5-µm sections were obtained from every pancreatic tissue (resected pancreatic tissue/biopsy or metastatic site biopsy). To study the expression of the exosomal markers (CD63 and CD9), immunohistochemical staining was performed. The unstained slides were first deparaffinized by using xylene (X1-10 min, X2-10 min) and subsequently hydrated by sequential incubation in sequential dilution of ethanol (100% EtOH 5 min, 70% EtOH 5 min, 50% EtOH 5 min, 30% EtOH 5 min, rinse the slides in running water 5 min). Thereafter, antigen retrieval was performed using Declokar chamber (Biocare Medical, Concord, CA, USA) with 1X Declokar buffer (Biocare Medical) followed by blocking of the endogenous peroxidase by incubation with peroxidase-1 (Biocare Medical). Later tissue sections were blocked with Background Sniper (Biocare Medical) for 10 min and incubated with the primary antibodies against CD63 and CD9 (1:50 and 1:25, respectively) (mouse monoclonal; Abcam, Cambridge, MA) overnight at 4 °C. Post-incubation, sections were washed and incubated with recommended polymer and probe (Biocare Medical) according to the manufacturer’s protocol. Immunoreactivity was visualized by DAB Chromogen followed by haematoxylin counterstain. Tissue sections incubated with normal mouse IgG (Santa Cruz Biotechnology Inc., Dallas, TX, USA) served as the negative control.
Scoring of CD63 and CD9 expression
Two pathologists independently scored the staining of CD63 and CD9. The intensity of cytoplasmic staining was graded from 1 to 3:1 represents (weak), 2 (moderate) and 3 (strong). The percentage of stained cells was estimated in 10% increments. A multiplicative Quick-score (Q-score) was calculated by multiplying the percentage of positive cells by the intensity of the staining (18). The average Q-score was calculated for each section.
Statistical analysis
The events defining progression free survival (PFS) and OS were death and recurrence/progression respectively. The OS time was the duration in months from the date of diagnosis until the date of death from any cause. Patients known to be alive were censored at the time of last contact. The PFS time was the duration in months from the date of diagnosis until the date of documented progression/recurrence or death, whichever happened earlier. The difference in exosomal markers (CD63 and CD9) expression between primary tumor and metastatic sites were compared and reported using independent sample t-test, and the results were further verified using Mann-Whitney U test and permutation test. Significance level of 0.05 was used to determine the significance of results. All tests were two-sided. We fitted Cox proportion hazard regression models to investigate the impact of the covariates CD63 and CD9 on PFS and OS. PFS and OS were estimated by the Kaplan-Meier and column scatter plots methods.
Results
Between October 2012 and December 2016, 49 patients with PDAC, treated at the University of South Alabama/Mitchell Cancer Institute, had available tissues [resected pancreatic tissue/biopsy (n=29) or metastatic site biopsy, liver, omentum or bone (n=20)] for CD63 and CD9 staining using IHC. The baseline characteristics of the patients are summarized in Table 1.

Full table
In patients with metastatic stage IV PDAC (n=20), the available tissue (biopsy) was from the metastatic site (liver, omentum or bone). In Patients with non-metastatic stage I, II and III PDAC (n=29), the available tissue was from the resected pancreas/pancreatic biopsy. The mean Q-scores for CD63 and CD9 are higher in the primary tumor from the pancreas compared to the pancreatic tumor from metastatic sites (185 vs. 102, P=0.0002) and (48 vs. 20, P=0.0418) respectively. Data is summarized in Table 2.

Full table
We fitted Cox proportion hazard regression models to investigate the impact of the covariates CD63 and CD9 on PFS and OS. CD63 has significant impact on PFS (P=0.0135) and OS (P=0.003). The higher the CD63 Q-score, the longer the PFS and OS. CD9 doesn’t have significant impact on PFS (P=0.5734) or OS (P=0.2682).
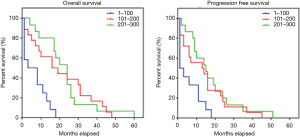
The median PFS and OS were longer in patients with PDAC with CD63 Q-score >100 compared to patients with CD63 Q-score of 100 or less. In patients with CD63 Q-score of 101–200 and patients with CD63 Q-score >200, the median PFS was 13 and 16 months respectively and the median OS was 24 and 24 months respectively. However, in patients with CD63 Q-score of 100 or less, the median PFS was 1 month and the median OS was 2 months. Figure 1 shows Kaplan-Meier curves for PFS and OS according to CD63 expression. Patients with PFS or OS of 6 months or less tend to have less expression of CD63 while patients with PFS or OS more than 6 months tend to have higher expression of CD63. Figure 2 shows column scatter plots demonstrating CD63 expression in patients with PDAC according to their PFS and OS.
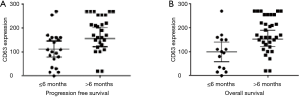
The mean CD63 and CD9 Q-scores are slightly higher in AA compared to Caucasians (157 vs. 149, P=0.76) and (45 vs. 29, P=0.43) respectively. However, Caucasian patients have significantly lower hazard than the AA patients and patients of other ethnicities in term of PFS (0.42:1.0, P=0.0429) and OS (0.24:0.10, P=0.002). Table 2 shows the mean CD63 and CD9 Q-scores according to patients’ ethnicity.
Discussion
PDAC-derived exosomes participate in many steps that are vital to cancer progression, metastasis, chemoresistance and immune modulation. That makes PDAC-derived exosomes potential biomarkers that might have prognostic significance.
The exosomal marker CD9 (originally called motility-related protein-1, MRP-1) is widely expressed glycoprotein in hematopoietic tissues and non-hematopoietic tissues (19). It has been suggested that low CD9 expression might be associated with metastatic potential. The expression of CD9 appears to diminish in the metastatic sites compared to the primary tumor in patients with breast (using IHC and immunoblotting) and colon (using differential display cloning) cancer (20,21). In patients with PDAC, the proportion of patients whose tumors had decreased CD9 expression increased from 14.3% of those with stage I to 85.7% of those with stage IV (15). In our study, the mean Q-score of CD9 expression using IHC was higher in the primary pancreatic tumor compared CD9 expression in the pancreatic tumor from metastatic sites (48 vs. 20, P=0.0418). The data from our cohort of patients with PDAC support the presumed notion that low CD9 expression might be associated with metastatic potential.
Similarly, the expression of CD63 is reported to be stronger in patients with early stages of malignant melanoma compared to patients with more advanced stages (22). In patients with PDAC, the expression of CD63 was positive in all cases (15). In our study, the mean Q-score of CD63 expression using IHC was higher in the primary pancreatic tumor compared CD63 expression in the pancreatic tumor from metastatic sites (185 vs. 102, P=0.0002). The data from our cohort of patients with PDAC show that there is differential expression of CD63 and its quantitative assessment is feasible. To our knowledge, our study is the first to show that decreased CD63 expression using IHC in patients with PDAC might be associated with metastatic potential.
Decreased CD9 expression was associated with poor prognosis in patients with breast cancer and lung cancer (21,23). However, no prognostic significance of CD9 expression, using IHC, was observed in patients with gastrointestinal stromal tumor (GIST) (24). In patients with PDAC, low CD9 expression had a worse 1-year survival and median OS compared to patients with high CD9 expression (0% vs. 25.5%, P=0.0004; 226 vs. 397 days, P=0.018) (15). In our study, the expression of CD9 was not associated with statistically significant prognostic value. The lack of statistical significance is likely due to technical difficulties with CD9 expression in the biopsies from metastatic sites. Staining for CD9 was performed twice on the biopsies from metastatic sites. Staining was faint and in many patients and in some patients the Q-score was zero. That was not exactly the case on the tissues from the resected pancreatic tissues, but still the Q-score was always not as strong as the expression for CD63. It is important to report that Sho et al. performed the CD9 IHC staining on frozen sections because of the notion that CD9 is not well preserved in FFPE (formalin-fixed, paraffin-embedded) specimens.
Increased expression of CD63, using IHC, was associated with poor prognosis in patients with GIST (24). In patients with PDAC, the expression of CD63 was reportedly positive in all patients without reported prognostic significance (15). In our study, CD63 expression has significant impact on PFS and OS. The higher the CD63 Q-score, the longer the PFS and OS. To our knowledge, our study is the first to show that CD63 expression using IHC in patients with PDAC has prognostic significance and correlate with PFS and OS outcomes.
Under hypoxia, both AA and Caucasian prostate cancer cells secrete higher number of exosomes (more so in African American) (25). The difference in exosomal markers expression between different racial backgrounds is yet to be fully explored. The reviewed literature that addressed CD9 and CD63 expression in colon cancer, breast cancer, melanoma and lung cancer did not report information about their cohort ethnic background (20-23). In one study that explored the expression of CD63 and CD9 in patients with GIST, the patient cohort was ethnically homogenous Caucasians. The studies that explored the expression of CD63 and CD9 in patients with PDAC did not report information about the ethnic background of the included patients but one study was done in Japan (14,15). In our cohort, the mean CD63 and CD9 Q-scores are slightly higher in AA compared to Caucasians (157 vs. 149, P=0.76) and (45 vs. 29, P=0.43), respectively. The reason the difference was not statistically significant is possibly because our cohort is small. Caucasian patients, despite having slightly lower CD63 and CD9 expression in our cohort, have significantly lower hazard than AA and patients of other ethnicities in term of PFS (0.42:1.0, P=0.0429) and OS (0.24:0.10, P=0.002). It is interesting to further study exosomes as a potential marker that could shed more light about the disparity in outcomes seen in different ethnic backgrounds.
Our study has several limitations. This study is a retrospective study and there are inherent limitations and selection bias associated with a retrospective analysis of this sort. It also represents a single institution experience and the sample size is relatively small. There is also significant heterogeneity in the patient population, including tumor stage, tumor location within the pancreas and the treatment considered by the treating oncologist. Moreover, although exosomes possess great potential as novel prognostic biomarkers, several challenges remain to be solved.
The exosomal markers (CD63 and CD9) detected using IHC likely indicate the presence of a diverse group of exosomes subtypes. In the context of cancer, it is critical to determine the origin of exosomes and be able to distinguish exosomes derived from tumor cells and exosomes derived from non-tumor cells. Tumor cells-derived exosomes possess unique contents and functions compared with non-tumor cells-derived exosomes (26,27). In our study, despite the positive correlation between CD63 expression and PFS and OS, it should be noted that studying the expression of CD63 is not enough to distinguish tumor cells-derived exosomes from non-tumor cells-derived exosomes.
Exosomes transfer various functional biomolecules including RNAs, DNA, lipids and proteins. The different contents of exosomes likely exhibit different effect on the recipient cell and hence different prognostic significance. In one study, it was suggested that macrophage migration inhibitory factor (MIF) could be used as a prognostic biomarker in PDAC. The level of macrophage MIF was higher in exosomes isolated from PDAC patients with disease progression compared to control healthy subjects and PDAC patients who exhibited no disease progression five years post diagnosis (9). In our study, despite the demonstrated different expression pattern of CD63 and its prognostic significance, the expression of CD63 does not provide information about the content of the exosomes.
Exosomes play a role in many steps that are vital to cancer progression, metastasis and chemoresistance but, at the same time, exosomes have immunomodulatory effects. Cancer cell-derived exosomes exhibit contradictory functions on the immune system. Some cancer cells secrete exosomes that could later be identified and captured by the immune systems, leading to immune-surveillance and anti-tumor activity (28-30). However, some cancer cells secrete exosomes that could suppress the function of many immune cells, leading to immune tolerance (28,30-32). In our study, despite the demonstrated different expression patterns of CD63 and its prognostic significance, the expression of CD63 does not provide information about the effect of exosomes on their recipient cells and especially the exact effect on the immune system.
Despite the prognostic significance that we were able to demonstrate, studies of larger cohorts and studies that are capable of addressing the limitations that we had in ours will likely provide more solid data about the prognostic significance of exosomes in patients with PDAC. Developing advanced protocols that are capable of purifying selected exosomes subtypes and contents is critical for precisely relating specific functions and physiological properties to a specific subtype and content of exosomes. Overall, our study should be considered hypothesis generating rather than definitive due to its limitations.
Conclusions
In summary, CD63 and CD9 expression is higher in primary tumor from the pancreas compared to pancreatic tumor from metastatic sites. There is correlation between CD63 expression (but not CD9 in this cohort) and PFS and OS. To our knowledge, this is the first study to show prognostic significance of CD63 expression in patients with PDAC using IHC. A trend of higher expression of CD63 and CD9 among AA compared to Caucasians was also noticed.
Acknowledgments
Funding: We would like to acknowledge the funding support from NIH/NCI [R01CA175772 and U01CA185490 (to AP Singh)] and USAMCI, and the funding support for the statistical analysis from the Simons Foundation (#422535, B Wang) and an award from the National Center for Advancing Sciences of the National Institutes of Health (UL1TR001417).
Footnote
Conflicts of Interest: Abstract published online at the American Society of Clinical Oncology (ASCO) 2017 meeting; presented as a poster at the ESMO World Congress on Gastrointestinal Cancer 2017 in Barcelona, Spain; presented as a poster at the GI ASCO meeting in San Francisco, CA in January 2018.
Ethical Statement: The University of South Alabama Institutional Review Board (IRB, No. 836682-4) approved this study and the IRB-approved database provided a waiver of the requirement for informed consent and allowed for publication of de-identified data.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Kang MJ, Jang JY, Chang YR, et al. Revisiting the concept of lymph node metastases of pancreatic head cancer: number of metastatic lymph nodes and lymph node ratio according to N stage. Ann Surg Oncol 2014;21:1545-51. [Crossref] [PubMed]
- Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol 2008;31:446-53. [Crossref] [PubMed]
- Ansari D, Rosendahl A, Elebro J, et al. Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. Br J Surg 2011;98:1041-55. [Crossref] [PubMed]
- Wald P, Liu XS, Pettit C, et al. Prognostic value of microRNA expression levels in pancreatic adenocarcinoma: a review of the literature. Oncotarget 2017;8:73345-61. [Crossref] [PubMed]
- Liao X, Huang K, Huang R, et al. Genome-scale analysis to identify prognostic markers in patients with early-stage pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Onco Targets Ther 2017;10:4493-506. [Crossref] [PubMed]
- Mathivanan S, Fahner CJ, Reid GE, et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 2012;40:D1241-4. [Crossref] [PubMed]
- Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470-6. [Crossref] [PubMed]
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816-26. [Crossref] [PubMed]
- Patel GK, Patton MC, Singh S, et al. Pancreatic Cancer Exosomes: Shedding Off for a Meaningful Journey. Pancreat Disord Ther 2016;6:e148. [Crossref] [PubMed]
- Patel GK, Khan MA, Bhardwaj A, et al. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br J Cancer 2017;116:609-19. [Crossref] [PubMed]
- Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 2011;33:441-54. [Crossref] [PubMed]
- Khushman M, Bhardwaj A, Patel GK, et al. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017;46:782-8. [Crossref] [PubMed]
- Gronborg M, Kristiansen TZ, Iwahori A, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics 2006;5:157-71. [Crossref] [PubMed]
- Sho M, Adachi M, Taki T, et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer 1998;79:509-16. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569-79. [Crossref] [PubMed]
- Detre S, Saclani Jotti G, Dowsett M. A. "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995;48:876-8. [Crossref] [PubMed]
- Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today 1994;15:588-94. [Crossref] [PubMed]
- Cajot JF, Sordat I, Silvestre T, et al. Differential display cloning identifies motility-related protein (MRP1/CD9) as highly expressed in primary compared to metastatic human colon carcinoma cells. Cancer Res 1997;57:2593-7. [PubMed]
- Miyake M, Nakano K, Ieki Y, et al. Motility related protein 1 (MRP-1/CD9) expression: inverse correlation with metastases in breast cancer. Cancer Res 1995;55:4127-31. [PubMed]
- Hotta H, Ross AH, Huebner K, et al. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res 1988;48:2955-62. [PubMed]
- Higashiyama M, Taki T, Ieki Y, et al. Reduced motility related protein-1 (MRP-1/CD9) gene expression as a factor of poor prognosis in non-small cell lung cancer. Cancer Res 1995;55:6040-4. [PubMed]
- Lewitowicz P, Matykiewicz J, Koziel D, et al. CD63 and GLUT-1 Overexpression Could Predict a Poor Clinical Outcome in GIST: A Study of 54 Cases with Follow-Up. Gastroenterol Res Pract 2016;2016:6478374. [Crossref] [PubMed]
- Panigrahi GK, Praharaj PP, Peak TC, et al. Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep 2018;8:3853. [Crossref] [PubMed]
- Heiler S, Wang Z, Zoller M. Pancreatic cancer stem cell markers and exosomes - the incentive push. World J Gastroenterol 2016;22:5971-6007. [Crossref] [PubMed]
- Wang Z, von Au A, Schnolzer M, et al. CD44v6-competent tumor exosomes promote motility, invasion and cancer-initiating cell marker expression in pancreatic and colorectal cancer cells. Oncotarget 2016;7:55409-36. [PubMed]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9:581-93. [Crossref] [PubMed]
- Zech D, Rana S, Buchler MW, et al. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal 2012;10:37. [Crossref] [PubMed]
- Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep 2016;6:20254. [Crossref] [PubMed]
- Bobrie A, Colombo M, Raposo G, et al. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 2011;12:1659-68. [Crossref] [PubMed]
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016;126:1216-23. [Crossref] [PubMed]


