Biliary and gastric drainage in advanced pancreatic serous cystadenoma and portal hypertension in Von Hippel-Lindau syndrome
Case introduction
A 41-year-old Hispanic female patient, with a pertinent past medical history of poorly-controlled diabetes mellitus, was referred with an established diagnosis of Von Hippel-Lindau syndrome (VHL) type II. The patient was complaining of 2 months of abdominal pain, associated with poor PO intake and 20 lbs weight loss with persistent nausea and vomiting. At presentation, the patient diabetes was poorly controlled on oral hypoglycemic and high dose of long acting insulin with HbA1c of 16.4%. Her liver function tests were perturbed with total bilirubin 1.5 mg/dL, alanine transaminase 521 units/L, aspartate transaminase 1,058 units/L and alkaline phosphatase 1,196 units/L. Hepatitis panel was non reactive.
On physical examination the abdomen was enlarged without fluid waves and minimally tender to palpation along the epigastrium/right upper quadrant with clear evidence of caput medusae.
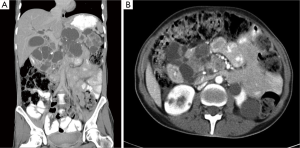
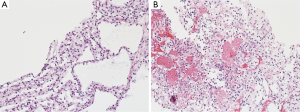
The radiological work up included an abdomino-pelvic computer tomography scan (CT), with iodide-based contrast. The reconstructed images revealed a large peri-pancreatic cystic tumor resulting in a mass effect obstructing the duodenal and the biliary system. There were severe hepato-billiary dilatation, gastric-outlet obstruction, and radiological evidence of portal hypertension with splenic vein and main portal vein obstruction and evidence of development of large portal collaterals. Additionally, the scan incriminated a left renal tumor without adjacent invasion (Figure 1). Chest CT and brain magnetic resonance imaging (MRI) were negative for pulmonary and cranio-cerebral pathology. Subsequent CT guided biopsy of the left renal tumor and the cystic pancreatic tumor revealed a clear renal cell carcinoma and a serous pancreatic cystadenoma, respectively, on final pathology (Figure 2). Although the standard diagnosis of VHL relies on genetic testing, the concomitant occurrence of the aforementioned malignancies is pathognomonic.
A staged operation was planned. First, as dictated by the patient symptoms, we prioritized the gastric and biliary drainage to be followed by partial left nephrectomy. Major pancreatic resection and dissection of the hepatic pedicle-common bile duct were limited by the concomitant portal hypertension. A gastric and biliary drainage consisting of gastrojejunostomy and cholecystojejunostomy was planned.
A mini laparotomy (10 cm) supra-umbilical incision was made and an antecolic anterior stapled gastro-jejunostomy was performed. A segment of proximal jejunum 20 cm from the ligament of Trietz was secured with interrupted 3-0 silk stiches to the anterior gastric wall and anastomosed side-to-side with 75 mm gastro-intestinal anastomosis (GIA-75) with the point of entry of the staple controlled and closed using 60 mm thoraco-abdominal (TA-60) blue load staplers. Along the same jejunal limb, 15 cm distal to the gastro-jejunostomy, a hand sewn cholecysto-jejunostomy was performed through an omentoplasty sleeve. The omental sleeve was performed to seal any potential biliary leak from the cholecystojejunostomy. After, the gallbladder was secured in position with non transfixing 5-0 polydioxanone (PDS), its inferior posterior aspect was opened for about 1.5 cm with electro-cautery allowing for bile aspiration and confection of the cholecysto-jejunostomy with a running 5-0 PDS. The anastomosis was internally stented using 8 Fr pediatric feeding tube. Finally, a sentinel 19 Fr. Blake drain was placed around the bilio-enteric anastomosis.
The patient had a smooth postoperative recovery with no morbidities and the diet was advanced gradually with immediate resolving of her preoperative nausea and vomiting. She was discharged home on the fifth postoperative day.
The Blake drain was removed during a clinic visit with near null output approximately one week from date of discharge. At one month postoperatively, the patient reported improved oral intake and 18 lbs weight gain. Furthermore, aspartate transaminase, alanine transaminase, total bilirubin, and alkaline phosphatase have all trended towards normalization (32, 29, 151 U/L, and 0.6 mg/dL, respectively) corroborating a successful hepato-biliary decompression. Notwithstanding unchanged home doses of metformin and long-acting insulin, and despite resumption of oral intake, average HgbA1c levels diminished from 16.4% to 11% at three months after the surgery. The patient underwent subsequently an uneventful partial left nephrectomy and recovered well from her second surgery.
Discussion
VHL was initially described by E. von Hippel (1) and A. Lindau (2) early in the 20th century. It is a rare highly penetrant autosomal dominant genetic predisposition to malignant and benign tumors, emanating from over 1,000 possible mutations in the VHL tumor suppressor gene on chromosome 3p25. However, approximately 20% of these mutations occur de novo (3).
It is typically associated with central nervous system hemangioblastomas, clear renal cell carcinomas, cystadenomas, and neuro-endocrine tumors depending on the sub-type (4). Patients with VHL type I usually manifest hemangioblastomas but rarely present with clear renal cell carcinoma or pheochromocytoma. On the other hand, patients with VHL type II comprises sub-types A, B, and C, which predispose to the development of hemangioblastoma; hemangioblastomas and clear renal cell carcinoma; and pheochromocytomas; respectively (4).
The rarely observed VHL type III is associated with Chuvash polycythemia. Pancreatic serous cystadenomas are entail a relatively rare VHL presentation, affecting only ~10% of patients (5). Further rarely, the above mentioned patient sub-population develops hepato-biliary obstruction requiring decompression (6).
In the presented case, the pancreatic serous cystadenomas (Figure 1) was unresectable as per the extent of the disease and the concomitant portal hypertension. Yet, a suitable palliative alternative was de rigueur. Percutaneous cholecytostomy tube (PTC) would transiently palliate hepato-billiary, but would not alleviate gastric outlet obstruction. PTC is inconvenient in a functional patient with favorable prognosis. Duodenal stent option was limited by the distorted anatomy and would not resolve the biliary obstruction. Ideally, this patient would require a choledocho- (or haepatico-) jejunostomy and a gastro-jejunostomy fashioned on a single limb or through a Roux-en-Y reconstructive bypass. Nevertheless, extensive portal-hypertension secondary to occlusion of the portal vein and the subsequent development of varices at the level of the hepatoduodenal ligament would render this surgical option unnecessarily risky, if not unwantedly morbid. Along the same line, any laparoscopic approach would be discouraging. As a result, a cholecysto-jejunostomy (in addition to a gastro-jejunostomy) was performed. Currently, this procedure turned into a mere historical curiosity—it is performed only as a last resort, and most often in the developing world where resources are scarce, expertise is scant, and patients generally present with advanced disease (7). However, this procedure constitutes a safe and effective last-resort in the hepato-billiary armamentary.
Undoubtedly, the surgical procedure described above does not treat portal hypertension. But since the patient remains asymptomatic, neither a surgical shunt nor a trans-jugular intra-hepatic systemic shunt is currently indicated. It is hard to explain the uncontrolled hyperglycemia experienced by the patient. The working hypothesis is that the hepato-biliary obstruction jeopardized pancreatic function through and inflammatory process. It is possible that the mitigating decompression permitted the pancreas to heal and the inflammation to subside explaining the improvement of her post-drainage glycemic control.
Conclusions
Hepato-billiary obstruction secondary to VHL-related pancreatic serous cystadenoma is extremely rare. To the knowledge of the authors, this is the only recent report, describing a palliative biliary decompression for VHL-related pancreatic serous cystadenoma with a cholecysto-jejunostomy. This approach successfully resolved the gastric outlet and the hepato-biliary obstruction resolved, and possibly ameliorated the patient glycemic control.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- von Hippel E.Über eine sehr seltene Erkrankung der Netzhaut. Albrecht von Graefes Archiv für Ophthalmologie 1904;59:83-106.
- Lindau A.Zur Frage der Angiomatosis Retinae und Ihrer Hirncomplikation. Acta Ophthal 1927;4:193-226.
- Frantzen C, Links TP, Giles RH. Von Hippel-Lindau Disease. 2000 May 17 (Updated 2012 Jun 21). In: Pagon RA, Adam MP, Bird TD, et al. eds. GeneReviews™ (Internet). Seattle (WA): University of Washington, Seattle;1993-2014. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1463/
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet 2003;361:2059-67. [PubMed]
- Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology 2000;119:1087-95. [PubMed]
- Gupta R, Chettri D, Sharma A, et al. Pancreatic cysts causing biliary obstruction in von Hippel Lindau syndrome. JOP 2008;9:313-6. [PubMed]
- Singh SM, Reber HA. Surgical palliation for pancreatic cancer. Surg Clin North Am 1989;69:599-611. [PubMed]


