Tumor response assessment in locally advanced colon cancer after neoadjuvant chemotherapy
Introduction
Colon cancer is a highly prevalent disease and the fourth most common cause of cancer death in western countries (1). The currently accepted standard of care in locally advanced colon cancer (LACC) is complete surgical excision followed by adjuvant chemotherapy. This approach achieves 5-year survival rates varying from 73% to 28%, depending on the stage III subgroup analyzed (2).
Several recent trials have been developed in order to assess the role of neoadjuvant chemotherapy in LACC (3-5), resembling its use in other locally advanced tumors (6,7). Besides the risk of tumor progression during the induction therapy, one of the most important challenges of this approach is the accuracy of baseline computed tomography (CT) scan to properly select patients who may benefit most from this strategy. The scarce available data in this setting is partly responsible for the lack of a more widespread use of neoadjuvant chemotherapy, despite its several theoretical benefits.
The aim of the present study is to assess the accuracy of CT scan in the staging of these patients and to correlate radiological, metabolic and pathological changes found after preoperative oxaliplatin and fluoropyrimidine-based chemotherapy.
Material and methods
The study included patients with LACC who completed preoperative chemotherapy and surgery within a tertiary center. Eligibility and exclusion criteria have been reported elsewhere (5). Eligibility criteria included age >18 years, diagnosis of adenocarcinoma by biopsy, Karnofsky performance status >60% or ECOG <2, Haemoglobin >10 g/dL, white blood cell >3.0×109/L, Total Bilirubin <25 mcromol/L, glomerular filtration rate >50 mL/min, absence of important comorbidity, and able and willing to provide written informed consent for the study. Radiological signs of suspicious lymph nodes and/or transmural depth invasion by CT were mandatory. Rectal tumours, distant metastases, peritoneal carcinomatosis by CT or positron emission tomography (PET)/CT scan, and complete colonic obstruction were considered exclusion criteria.
All patients received induction chemotherapy with oxaliplatin and capecitabine on a biweekly basis. The study protocol was approved by the Institutional Review Board.
The clinical staging was based on physical examination, colonoscopy with biopsy confirmation, and thoracoabdominopelvic CT scan. In fourteen patients, a whole-body 18Fluorodeoxyglucose (18FDG) PET-CT scan was also available.
CT scan protocol
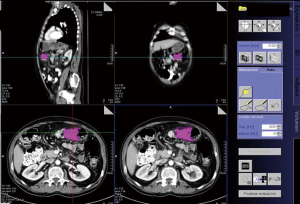
The patients were examined using a multidetector CT (MDCT) Scanner Siemens 64 (Erlangen, Germany). CT scans were obtained with a tube current of 150 mA and 120 kV of voltage with a 480 mm field of view and a 512×512 matrix. Oral contrast material [Diatrizoate sodium meglumine (Gastrografin; Bayer Hispania, SL, Saipan)] was administered before the study to achieve an adequate colonic opacification and distention. Intravenous non-ionic contrast agent (Omnipaque® 300 mg iodine/mL; Nycomed, Hispania, SL, Saipan) was also administered. The standard CT acquisition protocol was performed in the venous phase—start delay of 70 seconds—to maximize the detection of eventual hepatic metastases. A section of 5 mm width was performed. An intraluminal lesion with wall thickening but without surrounding tissue infiltration was defined as a T2 classification. Spiculated tissue extending from the colonic wall into the pericolic fat was characterized as a T3 classification. Colonic wall masses with infiltration of other surrounding organs were considered as T4 classification. Regional lymph node >0.8 cm in short axis diameter were considered pathologic. The tumor volume was measured using semiautomatic segmentation dedicated software (Volume Wizard®, Siemens Medical Solutions, Erlangen, Germany). In CT, absolute Hounsfield units (HU) correspond directly to the tissue property. Thus, a predefined soft tissue window display setting (300 to 45 HU) was applied to determine the tumor volume. The tumor was manually defined and segmentation of the entire scanning volume was performed automatically, with manual adjustments when necessary. The tumor was measured across the total imaging volume and calculated in cubic centimeters (cc). Figure 1 shows the tumor volume estimation.
PET-CT scan protocol
Patients were required to fast for 6 h. A blood glucose analysis was performed to ensure that levels were under 120 mg/dL. All patients received an intravenous injection of 6.29 MBq/kg of (18F)-FDG. One hour after the injection, (18F)-FDG-PET/CT studies were performed using a Biograph DUO scanner (Siemens, Knoxville, TN, USA). PET emission images were acquired with patients in a supine position using the 3-D mode (field of view 50 cm in the transaxial plane and 15.5 cm in the axial plane), at three min per bed position. CT data were used for attenuation correction and anatomical location of PET emission data. Quantitative measurement was normalized for the dose administered and the weight of the patients [standardized uptake value (SUV)].
Response evaluation
A CT (or PET-CT) scan was repeated 3-4 weeks after the end of neoadjuvant chemotherapy in order to assess tumour response and to confirm the resectability.
Radiologic response
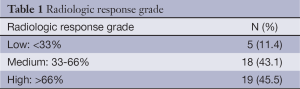
The percentual tumor volume differences were calculated by CT. Three response categories were established: minor (volume reduction <33%), medium (33-66%) and major (>66%). Changes in T and N classification were also recorded.
Metabolic response
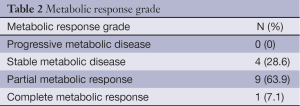
The results were categorized according to the definitions provided by the European Organisation for Research and Treatment of Cancer (EORTC) (8). Progressive metabolic disease was considered as an increase in [18F]-FDG tumor SUV of greater than 25% within the tumor region; stable metabolic disease as an increase in tumor [18F]-FDG SUV of less than 25% or a decrease of less than 25%; partial metabolic response as a reduction greater than 25% in tumor [18F]-FDG SUV; and complete metabolic response as the complete resolution of [18F]-FDG uptake within the tumor volume. Due to the necessity of bypassing chemotherapeutic effect and to avoid the fluctuation in 18F-FDG uptake that may occur early after treatment (stunning or flare of tumor uptake) a minimum of ten days after the end of chemotherapy was required before PET/TC performance (9).
Pathologic response
Pathologic staging was performed according to the TNM classification (2). Lymphovascular and perineural invasion, distal and circumferential margins were also documented. Tumor regression grade (TRG) was reported according to the scale proposed by Ruo et al. for rectal cancer (10). This classification considers 6 grades of response: grade 0 (no response to treatment), grade 1 (response <33%), grade 2 (response between 33% and 66%), grade 3 (response between 66% and 94%), grade 3+ (95-99% response, focus or microscopic residual), and grade 4 [no viable tumor identified, pathological complete response (PCR)].
Relationship between radiologic, metabolic and pathologic findings
Correlation between radiological and pathological findings was assessed in order to determine the predictive value of the CT scan after neoadjuvant treatment. Accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for T stage, N stage and for TN stage. Relationship between tumor volume changes by CT scan, SUV-FDG uptake by PET, and pathologic response were also analysed.
Statistical analysis
All the statistical analyses were done using the SPSS/PC v.15 for Windows statistical package (SPSS, Chicago, IL, USA). Results were expressed as mean (standard deviation) or median (P25-P75) for continuous variables depending on whether normal distribution was followed or not. Proportion was used for qualitative variables. Relationship between variables were studied by Student-t (or Mann-Whitney U, depending if data followed a normal distribution or did not) and χ2 tests. Student’s t or Wilcoxon test was also employed for paired samples. Association was measured by ANOVA and Spearman correlation. A P value <0.05 was considered significant.
Results
From July 2009 to June 2012, forty-four consecutive patients completed neoadjuvant treatment and underwent surgery. Median age was 66.8 years, 65.9% (29/44) of them were males and the mean BMI was 26.7 kg/m2 The most frequent tumor location was sigmoid colon (47.7%, 21/44) followed by ascending colon (34.1%, 15/44).
Radiologic response
Radiologic response was reported in the 42 patients (95.5%) in which pre- and post-treatment CT scan data was available. All patients achieved a tumor reduction, with no patient presenting metastatic progressive disease during the neoadjuvant chemotherapy. Median baseline tumor volume was 51.0 cc (range, 28.9-75.5 cc) compared to 18.4 cc (range, 8.7-30.3 cc) after chemotherapy. This translates into a statistically significant reduction of 62.5% (range, 38.3-81.8%) (P<0.001; Wilcoxon test). Table 1 shows the percentual tumor volume differences. The 61.9% (26/42) of the patients achieved a tumor volume reduction greater than 50%.
Full table
Metabolic response
Pre and post chemotherapy PET-CT scan was available in fourteen patients. Median baseline SUV value was 18.9 (range, 13.1-24) compared to 10.7 (range, 5.3-15.6) after treatment, for a median reduction of 38.9% (range, 9.6-63.7%) (P=0.004; Wilcoxon test). The median interval between the end of chemotherapy and the pre-surgery PET/CT was 21.5 days (range, 16.8-22 days). As shown in Table 2, >70% (10/14) of the patients achieved a metabolic response.
Full table
Pathologic response
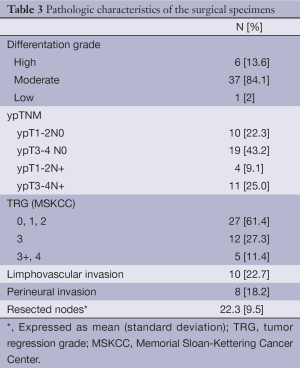
Table 3 summarizes the pathological findings of our study. Stage II and III disease was observed in 29/44 (65.9%) and 15/44 (34.1%) of the patients, respectively. Pathologic complete response was achieved in three patients. 38.7% (17/44) of the patients achieved a grade 3 or greater TRG. Mean number of harvested nodes was 22.3 (9.5). Disease free resection margins were obtained in all cases.
Full table
Radiologic and pathologic relation T classification relation
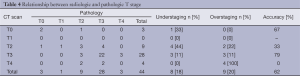
We first aimed to find the relation between the T classification, classified by CT scan after chemotherapy, and the T classification depicted in the final pathogic report. As seen in Table 4, accuracy was 62% (27/44), with an understaging rate of 18% (8/44) and an overstaging rate of 20.4% (9/44).
Full table
T0-2, were considered as Low T and T3-4, as High T. CT scan sensitivity, specificity, PPV and NPV for T classification were 87.1% (27/31), 61.6% (8/13), 84.4% (27/32) and 66.7% (8/12), respectively.
N stage correlation
Secondly, a correlation between CT scan and pathologic report was assayed. As shown in Table 5, accuracy for N classification was 87% (38/44), with a 5% (2/44) rate of understaging and a 9.1% (4/44) rate of overstaging.
CT scan sensitivity, specificity, PPV and NPV for N classification were 75.0% (12/16), 92.9% (26/28), 85.7% (12/14) and 86.7% (26/30), respectively, with a likelihood ratio of 10.6.

Full table
TN classification correlation
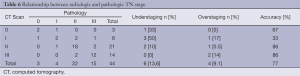
As shown in Table 6, accuracy for TN classification was 77.3% (34/44), with an under- and overstaging rate of 13.6% (6/44) and 9.1% (4/44), respectively.
Full table
Relation between clinical and pathologic TRG
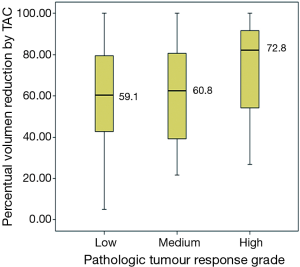
Correlation between the continuous percentual volume changes by CT scan before and after chemotherapy and the pathologic TRG (MSKCC score) was calculated by means of ANOVA test, after assaying normality of residuals. An association was found without reaching statistical significance (P=0.58) Figure 2.
Correlation between metabolic response by PET/CT scan and pathologic TRG was also studied. A positive but poor correlation was observed (rho =0.32; Spearman test) and without statistical significance (P=0.25).
Discussion
Currently, one of the most intriguing challenges in the management of patients with LACC is the way to select those who may benefit most from a neoadjuvant strategy. In this sense, the accuracy of imaging seems critical. Reported accuracy rates for CT in the preoperative staging of colon cancer range between 41% and 82% (11-14). In recent years, the use of oral and rectal contrast agents has improved the determination of the depth of invasion through the colonic wall, and MDCT has provided the additional capability of using thin collimation that offer an improved quality of MPRs and better spatial resolution. Despite thin sections, however, an intrinsic limitation of CT is the lack of visualization of the individual wall layers. The sensitivity of CT in detection of primary colon cancer is variable and depends on the size of the tumor. In this study we have found an accuracy of 62% (27/44) for T stage, within the range of that reported in the literature (15-17).
Another limitation of the CT staging relies on its inability to definitively distinguish metastatic lymph nodes. Small nodes may harbor tumor, and enlarged nodes may not. As expected, the sensitivity of CT for detection of malignant nodes decreased when applying the 1-cm threshold instead of a 0.8-cm threshold. Accuracy rates for N stage in recent papers range between 22% and 77% (17-19). In the present work, an accuracy of 87% (38/44) was achieved for N stage.
18F-FDG PET is a molecular imaging technique that visualizes and quantifies metabolic processes in cancer cells. PET has experienced an explosive growth as a diagnostic modality, especially in the realm of oncology for tumor staging, restaging, surveillance of recurrence and monitoring treatment response (20-22). PET/CT scans provide fused functional and morphological imaging, overcoming the lack of anatomical information of FDG-PET. There is an increasing interest in the role of FDG-PET for the prediction of tumor response to therapy, as it has been shown in lymphomas and esophageal cancer (23-26). Predictors of response in LACC are eagerly awaited due to the presumed favorable prognostic value of a complete response to neoadjuvant therapy in terms of improved survival times and the possibility of performing less aggressive surgical approaches (27-29).
Neoadjuvant strategies require highly accurate diagnostic tests for a proper selection of candidate patients (18-30). The reported accuracy for TN classification of 77%, with a low rate of overstaging, suggests that patient selection for neoadjuvant chemotherapy on the basis of currently available image techniques is promising. Nevertheless, prospective trials to rule out the best imaging workout to be performed with the highest accuracy seem warranted.
This study found a trend toward statistical significance in the relationship between the change in tumor volume measured by CT and the degree of pathological regression. This absence of significance might be due to the lack of statistical power. Similar results were found when assessing the correlation between metabolic and pathological response. These findings might allow considering the PET/TC to be a predictor tool of pathological response in the LACC in the future.
This study has some limitations that deserve consideration. This is a single institutional experience and recorded in a retrospective way. On the other hand we had few patients, which obligate the results to be handled with caution.
Conclusions
Neoadjuvant chemotherapy based on oxaliplatin and capecitabine for LACC induces a significative tumor response that can be measured at radiologic, metabolic and pathologic level. The accuracy and the low overstaging of CT scan may allow LACC patients to benefit from a neoadjuvant therapy with a low risk of overtreatment.
Acknowledgements
Authorship and contributions: HJ, AJ and MP contributed to study conception and design; GI, RM and SJ contributed to acquisition of data; RJ, PC and BJ contributed to analysis and interpretation of data and AJ and RJ wrote the manuscript. All the authors agreed on the final version. The manuscript is not being considered by another journal.
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Edge S, Byrd DR, Compton CC, et al. eds. AJCC Cancer Staging Manual. 7th ed. 2010.
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [PubMed]
- Memorial Sloan-Kettering Cancer Center. Neoadjuvant FOLFOX Plus Bevacizumab Chemotherapy in Patients With Locally Advanced Colon Cancer. Available online: http://clinicaltrials.gov/ct2/show/NCT00826800
- Arredondo J, Pastor C, Baixauli J, et al. Preliminary outcome of a treatment strategy based on perioperative chemotherapy and surgery in patients with locally advanced colon cancer. Colorectal Dis 2013;15:552-7. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [PubMed]
- Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773-82. [PubMed]
- Dehdashti F, Flanagan FL, Mortimer JE, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med 1999;26:51-6. [PubMed]
- Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 2002;236:75-81. [PubMed]
- McAndrew MR, Saba AK. Efficacy of routine preoperative computed tomography scans in colon cancer. Am Surg 1999;65:205-8. [PubMed]
- Barton JB, Langdale LA, Cummins JS, et al. The utility of routine preoperative computed tomography scanning in the management of veterans with colon cancer. Am J Surg 2002;183:499-503. [PubMed]
- Gollub MJ, Schwartz LH, Akhurst T. Update on colorectal cancer imaging. Radiol Clin North Am 2007;45:85-118. [PubMed]
- Chiesura-Corona M, Muzzio PC, Giust G, et al. Rectal cancer: CT local staging with histopathologic correlation. Abdom Imaging 2001;26:134-8. [PubMed]
- Dighe S, Swift I, Brown G. CT staging of colon cancer. Clin Radiol 2008;63:1372-9. [PubMed]
- Ashraf K, Ashraf O, Haider Z, et al. Colorectal carcinoma, preoperative evaluation by spiral computed tomography. J Pak Med Assoc 2006;56:149-53. [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [PubMed]
- Dighe S, Purkayastha S, Swift I, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol 2010;65:708-19. [PubMed]
- Kanamoto T, Matsuki M, Okuda J, et al. Preoperative evaluation of local invasion and metastatic lymph nodes of colorectal cancer and mesenteric vascular variations using multidetector-row computed tomography before laparoscopic surgery. J Comput Assist Tomogr 2007;31:831-9. [PubMed]
- Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008;49:480-508. [PubMed]
- Furukawa H, Ikuma H, Seki A, et al. Positron emission tomography scanning is not superior to whole body multidetector helical computed tomography in the preoperative staging of colorectal cancer. Gut 2006;55:1007-11. [PubMed]
- Zervos EE, Badgwell BD, Burak WE Jr, et al. Fluorodeoxyglucose positron emission tomography as an adjunct to carcinoembryonic antigen in the management of patients with presumed recurrent colorectal cancer and nondiagnostic radiologic workup. Surgery 2001;130:636-43; discussion 643-4. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [PubMed]
- Habr-Gama A, Perez R, Proscurshim I, et al. Complete clinical response after neoadjuvant chemoradiation for distal rectal cancer. Surg Oncol Clin N Am 2010;19:829-45. [PubMed]
- Smith NJ, Bees N, Barbachano Y, et al. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer 2007;96:1030-6. [PubMed]
- Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med 2007;48:36S-44S. [PubMed]
- Guillem JG, Moore HG, Akhurst T, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg 2004;199:1-7. [PubMed]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50:122S-50S. [PubMed]
- Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 2005;11:2785-808. [PubMed]
- Dighe S, Blake H, Koh MD, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg 2010;97:1407-15. [PubMed]