The value of oxaliplatin in the systemic treatment of locally advanced rectal cancer
Introduction
Locally advanced rectal cancer (LARC) is a heterogeneous disease in its molecular expression, cell differentiation, tissue invasion and intrapelvic anatomy, presenting two distinct challenges in the clinical practice of quality: (I) promotion of local tumor control in the pelvic region (minimizing the risk of loco-regional recurrence and favoring surgical strategies of anorectal sphincter complex preservation); and (II) promotion of systemic control of micrometastatic (subclinical) disease potentially present at the initial diagnosis.
For many years, the multidisciplinary approach of neoadjuvant radiotherapy (short-course and hypofractionated) with or without concurrent chemotherapy, followed by total excision of the mesorectum (surgery) and adjuvant chemotherapy with fluoropyrimidines has been accepted as the standard approach to cancers of locally advanced rectum (1). With this approach, local recurrence rates have fallen from 30% to 15% (2,3). The updated recommendation for patients with LARC stages II–III is treatment with chemoradiation (CRT) followed by surgery with total mesorectal excision (TME) and, subsequently, four to six cycles of adjuvant chemotherapy. To the date, we have no studies on this and the choice of adjuvant treatment is based on the response obtained after neoadjuvant treatment and the availability of effective drugs.
Based on the results of clinical trials, patients with cT3–T4 or cN+ rectal tumors are being treated, prior to surgery, with radiation and chemotherapy, mainly with fluoropyrimidines. However, the incidence of distant metastasis remains high, around 30%. Thus, combination chemotherapy regimens have been tried, similar to those used in metastatic disease, such as the addition of oxaliplatin and irinotecan, in order to improve the prognosis of patients with rectal cancer. It is suggested that combination chemotherapy with oxaliplatin could improve local control of the tumor in patients with resectable rectal cancer, although this regimen also causes greater toxicity (4).
Oxaliplatin has been shown to sensitize human cancer cells to the effects of in vitro radiation and to improve the prognosis of patients with metastatic colorectal cancer and locally advanced colon cancer, treated after surgery with adjuvant based on fluoropyrimidine plus oxaliplatin is added (5-7). Disease-free survival (DFS) improved by 23% and 26% in two large randomized trials that evaluated the addition of oxaliplatin to either the biweekly 5-fluorouracil (FU) infusion or the weekly 5-FU bolus (5,8).
The line of clinical research in healthcare innovation that analyzes this work in depth regarding the potential contribution of oxaliplatin as a therapeutic component was initially analyzed by Calvo et al. This is an analysis based on the care practice of the General University Hospital Gregorio Marañón, which incorporates oxaliplatin in two different therapeutic segments: neoadjuvance or adjuvance (9). In this analysis, with a retrospective design in a cohort of 207 consecutive patients, who received FOLFOX-4 induction in neoadjuvant, surgery was performed 6 weeks (range, 3–12 weeks) after chemoradiotherapy. It was concluded that the short and intense induction of FOLFOX-4 significantly improves downstaging and favors the preservation of the sphincter in low rectal tumors. With the criterion of extending to clinical practice therapeutic innovations with oncological value, oxaliplatin has been incorporated into a strategy adapted to the risk of LARC.
In the context of the biological heterogeneity of cancer currently accepted, there is an oxaliplatin-sensitive cell population in metastatic colorectal cancer sufficiently relevant to achieve a high response rate with this drug (the most active known), which has led us to undertake this work, in a context of innovated multidisciplinary practice, to evaluate whether the cumulative dose of oxaliplatin in adjuvant administration, interacts in terms of prognosis with other clinical and therapeutic factors, in the heterogeneous model of LARC, which is characterized by a dominant risk pattern of systematic progression.
Methods
Selection of patients
The study population consisted of patients with confirmed histological diagnosis of locally advanced adenocarcinoma of the rectum (cT3–4, cNxN0/+, cM0) referred to the General University Hospital Gregorio Marañón (Radiation Oncology Service), candidates for a radical treatment with a neoadjuvant component, surgical resection with intraoperative radiotherapy (IORT), followed or not by adjuvant chemotherapy according to a risk-adapted individualized recommendation. The data collection of the patients began in March 2013 and was completed in September 2015. A total of 212 patients were registered, of which 110 patients were in the neoadjuvant CT treatment group with oxaliplatin and 102 patients in the treatment group of neoadjuvant CT and adjuvant CT with oxaliplatin. The duration of the global follow-up of patients in the study was of an average of 72 months (6 years).
Diagnosis and staging
The endoscopic study (recto-sigmoidoscopy and/or colonoscopy) with biopsy and histological confirmation was an obligatory requirement in all patients. With this test the location and extension of the tumor with respect to the anal margin was determined and the presence of synchronous tumors in the colon was ruled out.
The staging of the tumor lesion and the lymph node clinical stage were evaluated by computerized tomography (CT) and endorectal ultrasound (ERUS). Other diagnostic methods such as nuclear magnetic resonance (NMR) or positron emission tomography (PET) were performed optionally in some cases to confirm the tumor stage, since they provide more elaborate information including morphological changes and metabolic changes (10-12). Once the segment of pre-operative treatment and prior to surgery was completed, around 4–6 weeks after the end of chemo-irradiation, patients were re-staged by endoscopic ultrasonography and thoraco-pelvic CT to evaluate the degree of clinical response obtained with neoadjuvant and rule out the presence of local or distant progression.
Description of the treatment and exposure
All patients underwent two cycles of chemotherapy regimen with FOLFOX-4 (oxaliplatin, 5-FU, and leucovorin) before radiotherapy and surgery, followed by adjuvant chemotherapy with FOLFOX-4 (oxaliplatin, 5-FU, and leucovorin), only for certain patients (according to recommendations adapted to the pathological and biological risk). The evaluation of the efficacy of oxaliplatin was carried out in the context of the total number of cycles received, that is, the total dose of drug administered, in the period of neoadjuvant treatment and in the adjuvant period for those patients who received it. Two groups of patients were determined according to the intensity and sequence of treatment of oxaliplatin received.
The dose of radiotherapy prescribed was 4,500 cGy for all the initial planning target volume (PTV), and 5,040 cGy on the macroscopic rectal tumor with margin of safety, administered in 28 fractions, during 6 weeks of treatment, using a daily fractionation of 180 cGy per day, 5 days a week, from Monday to Friday. During the irradiation tegafur was prescribed to all patients. The dose of tegafur was 1,200 mg/day orally, distributed in doses of 400 mg three times a day, throughout the irradiation period, including weekends. Radical surgical resection was scheduled 4–6 weeks after the end of the neoadjuvant chemo-irradiation segment. The protocol did not pre-establish the type of surgical procedure that had to be performed in each case (low anterior resection, abdominal-perineal amputation, endoanal resection) as long as the oncological margins of resection were assured with sufficient safety. Likewise, the performance of TME was not required, leaving it to the criteria of each participating surgical team, although it was considered a highly recommendable therapeutic element. IORT over the presacral region was incorporated as a component of selective overprinting over this area of high risk of recurrence.
All patients with pathological evidence of residual disease were considered candidates for postoperative chemotherapy, while in patients with complete pathological response, postoperative chemotherapy could be omitted at the discretion of the attending physician. Thus, patients with anatomopathological evidence of disease and treated with adjuvant CT were included in the cohort of patients with adjuvant CT using chemotherapy schemes that include oxaliplatin (FOLFOX-4).
Diagnosis and pathological evaluation
A criterion of most importance was the pathological staging using the TNM (tumor-node-metastasis cancer staging system) classification (13), and the tumor regression grade (TRG) reclassification scale proposed by Rödel et al. (14) was used and it ranges from TRG 4 when there are no viable tumor cells, to TRG 0, when there is no fibrosis. TRG 3 is defined as regression >50% with areas of fibrosis that exceed the tumor mass, TRG 2 is defined as regression <50%, and TRG 1 is basically defined as a morphologically unchanged tumor mass. In this study a classification was made in subgroups of patients after analyzing the results of TRG after receiving the neoadjuvant treatment scheme. Thus, patients with TRG 3–4 values were named as responders and those with TRG 1–2 values as non-responders.
Methods for statistical analysis
Initially, a preliminary descriptive analysis of the homogeneity of the characteristics of the patients included in both treatment groups was performed to detect the existence of statistically significant differences between the two cohorts that could generate systematic errors. Subsequently, an inferential analysis was carried out using univariate logistic regression of the clinical, pathological and therapeutic factors that could influence the pathological response and survival. The variables that showed significant differences in the univariate analysis were analyzed using the multiple factor analysis Cox method. The calculation of survival probability was carried out using the Kaplan-Meier method. The comparison of survival between groups based on different prognostic factors was performed using the logarithmic rank test (log-rank test). The level of statistical significance that was established was P<0.05, and the confidence interval (CI) of 95%. The statistical package used for the analysis of the data was the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the two cohorts
In this study 212 patients were included, 42% of women and 58% of men, with a median of 64.9 years (32–83 years). This distribution was maintained when comparing both treatment groups, presenting a median age, as well as a similar proportion of men and women among patients treated with oxaliplatin in neoadjuvance and those treated with oxaliplatin in neoadjuvance + adjuvance (P=0.240).
All the subjects included in the study presented the histological type of adenocarcinoma. More than half of the patients (64%) showed a histological G2 (moderately differentiated), 30% were G1 (well differentiated) and only 6% were G3 (poorly differentiated). These proportions remained comparable in both treatment groups, with no statistically significant differences (P=0.980).
The distribution of clinical stages T differed between the two treatment groups, with a higher proportion of cT4 in the (neoadjuvant + adjuvant)-oxaliplatin group (27% vs. 12%), with statistically significant differences (P=0.006).
Regarding lymph node affectation, a significantly higher proportion of patients evaluated by NMR were detected (68.70% vs. 21.7%) than by PET (2% vs. 0.9%), in favor of the oxaliplatin in neoadjuvant + adjuvant treatment group. Clinical nodal affection at diagnosis showed a higher proportion of patients in the (neoadjuvant + adjuvant)-oxaliplatin group (92.90% vs. 71.4%), with the difference being significant (P=0.000).
A clearly higher percentage of patients in stage III was observed in the (neoadjuvant + adjuvant)-oxaliplatin group compared to the (neoadjuvant)-oxaliplatin group (92.8% vs. 72.1%, P=0.000). The absence of a homogeneous distribution of the variable cN+ and cT4 between both groups (92.90% vs. 71.40%) and (27% vs. 12%), respectively, due to their potential prognostic implications in the results of the study in relation to the influence of the oxaliplatin dose on the pathological response, was taken into account in the inferential analysis phase including the multivariate analysis models, with the aim of minimizing possible selection biases.
The incidence and severity of adverse reactions recorded during pre-operative induction and radiochemotherapy treatment showed no relevant differences between both groups. During the preoperative radio-chemotherapy and the postoperative period, the patients had weekly clinical control in order to detect serious toxic effects (grades III–IV) according to the Common Terminology Criteria for Adverse Events (CTCAE) scale of the National Cancer Institute (NCI). Fifty-seven patients (36%) had some type of serious adverse effect: cutaneous toxicity in 19 patients (12%); gastrointestinal in 35 patients (22.1%); urinary in 1 patient (0.63%); and neutropenia of grade III in 2 patients (1.26%). These adverse events were reversible in all cases and resolved with symptomatic and supportive treatment. No deaths were documented due to toxicity.
The mean total dose of oxaliplatin administered in adjuvant chemotherapy was 527.48 mg/m2, with a wide range of doses (from a minimum of 83.66 to a maximum of 886.66 mg/m2) due to the variability of the total cycles that each patient received. The mean total dose of oxaliplatin administered in neoadjuvant + adjuvant chemotherapy was 420.76 mg/m2.
Pathological staging
The pathological staging of the entire series of patients was: 46 cases (21.8%) with stage ypN+ and 165 cases (78.2%) with stage ypN0. This means that after treatment with preoperative chemoradiation, about 80% of patients reached a state of absence of metastatic lymph node disease in a dominant manner. The absence of a homogeneous distribution (P=0.047) of the variable ypT0 and the variable ypT4 between both groups (16.4% vs. 5.9% and 1.8% vs. 5.9%, respectively) due to their potential involvement in the study results in relation to the efficacy of adjuvant treatment of oxaliplatin were taken into account in the inferential analysis phase in the multivariate analysis models, with the objective of minimizing possible selection biases. According to the TRG scale, the distribution of the different categories of pathological response was significantly different between the two series of patients, presenting a percentage of cases with minimum (TRG 1-2), intense response (TRG 3) and complete response (TRG 4) of 45%, 38.5% and 16.5% respectively, for the group of oxaliplatin in neoadjuvant and for the group of patients treated with oxaliplatin in neoadjuvant + adjuvant (57.4%, 36.6% and 5.9%, respectively) (P=0.049), which is interpreted as a post-neoadjuvant selection bias.
Univariate and multivariate analyses of the response percentages
The univariate and multivariate analyses showed that the dose of oxaliplatin in adjuvance (postoperative) greater than 6 cycles was positively associated with local recurrence-free survival (LRC) at 5 years (P=0.012, Figure 1) and with overall survival (OS) at 5 years (P=0.048, Figure 2). In the cohort of responding patients to neoadjuvant with oxaliplatin (with TRG 3–4), the dose of oxaliplatin in adjuvance (postoperative) greater than 5 cycles was positively associated with OS (P=0.06). And the dose of oxaliplatin in adjuvance (postoperative) in the range of 4–5 cycles was positively associated with the distant metastasis-free survival (DMFS) in the cohort of responding patients (P=0.015) and with DFS in the cohort of responding patients (P=0.004).
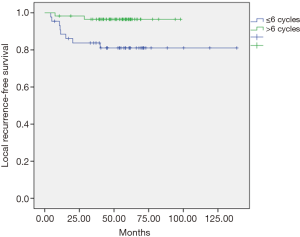
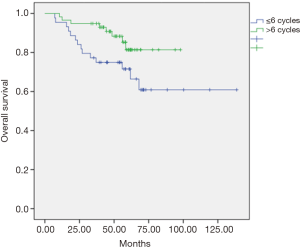
The results of both the univariate analysis and multivariate analysis in relation to response categories (responding patients) of LRC, DMFS, DFS and OS, and dose of oxaliplatin, are presented in Tables 1,2.
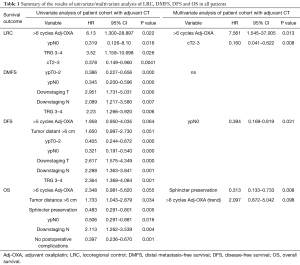
Full table
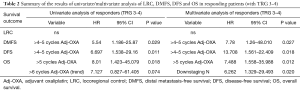
Full table
Discussion
The majority of patients who received oxaliplatin had a histological grade of adenocarcinoma G2 in similar percentages in the neoadjuvant treatment group vs. the neoadjuvant + adjuvant treatment group (64% vs. 63%), with an equally predominant location in the middle rectum (59% vs. 63%). However, there were significant differences in their clinical staging, probably due to a temporal selection bias in the study period. Number of patients in the clinical stage cT4 was higher in the oxaliplatin group in neoadjuvance + adjuvance compared to the group that received only neoadjuvant treatment (27% vs. 12%). Likewise, the stage cN+ (92.90% vs. 71.4%) and consequently the stage III with lymph node affection were more predominant in the neoadjuvant group (92.8% vs. 72.1%).
The interesting post-neoadjuvant elements were the observed pathological results: ypT4 (1.8% vs. 5.9%), ypN+ (18% vs. 25%), downstaging T (70% vs. 60%), downstaging N (55% vs. 67%) and pathologic complete response pCR (ypT0) (16% vs. 5.9%) with statistically significant differences. These results suggest that patients who had received neoadjuvant treatment and reached pCR were not selected for adjuvance with oxaliplatin because they were considered to be extreme responders. In reference to the TRG established, there were also statistically significant differences between the groups in the TRG 4 with values of 16% vs. 5.9%, respectively (P=0.049).
The resectability rates of the surgical specimen (R0) were high in both groups after the advanced excision techniques (more than 40% with laparoscopic approach), reaching percentages of 99% vs. 95%, achieving surgery with preservation of the anal sphincter of a 70% vs. 71%, respectively.
Therefore, in the descriptive analysis of both patient cohorts we can affirm that their sociodemographic, clinical and diagnostic characteristics have been very similar, given the inclusion of these patients based on selection criteria. On the other hand, they have marked significant differences in terms of their therapeutic selection due to the elements of adversity in the pathological staging after receiving the neoadjuvant treatment.
The results published after analyzing the subgroup of patients treated with preoperative chemo-irradiation in the German study CAO/ARO/AIO-94 (15), in terms of prognosis, suggest that obtaining pCR (TRG 4) would be related to a significant increase in DFS (at 5 years, 86%), compared to those cases that presented a moderate response (TRG 2–3) or no response (TRG 0–1), with DFS at 5 years of 75% and 63%, respectively (P=0.006). The data are similar for 5-years in distal metastasis-free survival (DMFS), which was 86%, 75%, and 66%, respectively (P=0.009). In our series of patients, the TRG found were: TRG 4 (11.40%), TRG 3 (37.60%), TRG 2 (43.80%) and TRG 1 (7.10%). In reference to DFS at 5 years, it was 80.70% for TRG 3–4 and 62% for TRG 1–2 (P=0.002), following the criterion of grouping into categories of intense or weak responders. Therefore, after the success obtained by the combination of CT with oxaliplatin as a neoadjuvant treatment in the increase of pCR and without excess of metastasis, it could be reasonable to administer oxaliplatin and to propose its use as a postoperative regimen. However, no trial has shown an improvement in OS.
Our study had the limitation of being retrospective, however it is a long-term follow-up analysis (over 6 years) with the combination of FOLFOX in induction, CT-RT, surgery and FOLFOX in adjuvant, from which not only an improvement in pCR has been obtained, but it has also demonstrated a potential to achieve higher survival rates, including DFS. Nevertheless, the benefit of adjuvant chemotherapy in patients who have received preoperative radiotherapy is unclear and this question has been reviewed and analyzed through two large meta-analyzes that include randomized trials.
The publication of a recent meta-analysis (16) of four randomized trials I-CNR-RT (17), CHRONICLE (18), PROCTOR-SCRIPT (19) and EORTC 22921 (20) showed that the differences in survival between patients who received postoperative chemotherapy and those who did not receive were not statistically significant. The meta-analysis of individual patient data ensures a lower risk of bias and allows the analysis of subgroups. This meta-analysis compares adjuvant chemotherapy with observation in patients who received pre-operative radiotherapy (16). The mean follow-up was 7 years. This meta-analysis is the only one published to date that has compared patients with rectal cancer who received preoperative chemoradiotherapy and fluoropyrimidine only in the postoperative period with patients who received combination chemotherapy (fluoropyrimidine-oxaliplatin) after surgery. The differences in DFS between the two groups were not statistically significant. Therefore, the evidence from randomized trials does not show that postoperative chemotherapy improves survival in patients with rectal cancer who have received pre-operative radiochemotherapy. However, the limitations of meta-analyzes must be recognized. The differences in the design of the trials should be taken into account in the interpretation of the effects of postoperative chemotherapy. In relation to our study, no statistically significant differences were found between patients who were only treated with neoadjuvant therapy versus those who were treated with neoadjuvant and adjuvant therapy in locoregional control (LRC) (91.40% vs. 89.80%), and neither in DFS (72% vs. 67%) nor in OS (77% vs. 78%) and, finally, neither in the DMFS (73% vs. 72%). In the population subgroups, according to whether they were responders or non-responders, they also showed no significant difference between the subgroups. This equivalence in results may indicate a risk compensation effect, just as our cohorts were selected. The group treated with neoadjuvant + adjuvant chemotherapy has a significantly higher rate of risk patients than the one treated with oxaliplatin in neoadjuvant only (TRG 1-2 57% vs. 45%, and pN+ 25% vs. 18%, not downstaging T 40% vs. 29%). In the oxaliplatin group only in neoadjuvant, non-responders have a significantly more adverse pattern compared to the responders (63% vs. 82% DFS, P=0.02), and the same for ypN+ versus ypN0 (54% vs. 80% SLE, P=0.06) and not downstaging T versus downstaging T (80% vs. 53% DFS, P=0.002). Similarly, in relation to OS, non-responders have a more adverse developmental pattern compared to responders (76% vs. 79% OS), and ypN+ vs. ypN0 (68% vs. 80% OS) and not downstaging T vs. downstaging T (67% vs. 80% OS). It can be hypothesized that adjuvant oxaliplatin compensates for the excess risk posed by unbalanced selection in its group of non-responders, to generate an evolutionary equivalence. Our study does not show that postoperative chemotherapy improves survival in patients with rectal cancer who received preoperative radiochemotherapy, as it has not been demonstrated in previous trials, although it suffers from a selection bias towards higher oncological risk that can be interpreted as compensation effect. As for the type of adjuvant chemotherapy that should be administered, currently there is no general consensus, and a commonly used scheme is the weekly administration of 5-FU and leucovorin (500 mg/m2 for 6 weeks) (21), although in many centers 5-FU monotherapy is still used. The recommendations from the most influential experts about the adjuvant treatment of LARC are indicated in the 2015 NCCN guidelines (Clinical Practice Guidelines in Oncology from the National Comprehensive Cancer Network).
In our study, the adjuvant chemotherapy treatment scheme was used with cycles of induction chemotherapy with FOLFOX-4 (oxaliplatin, 5-FU and leucovorin) administered every month or every month and a half. Although no significant differences were found between those who received and did not receive any adjuvant cycle, in view of our objectives we analyzed the effect of the total dose of oxaliplatin administered in the cohort of patients with adjuvant therapy based on the number of cycles received, finding some statistically significant differences. Thus, the dose of oxaliplatin in adjuvant over 6 cycles was positively associated with local recurrence-free survival (96% vs. 81%, P=0.012), and in reference to OS (81% vs. 72%, P=0.048) at 5 years it was found a tendency to increase with, equally, a dose of oxaliplatin greater than 6 cycles in the entire series of patients. Likewise, in patient responders with TRG 3-4, the effect of the total dose of oxaliplatin administered in this subgroup of patients with adjuvant therapy was analyzed based on the number of cycles received, finding also statistically significant differences. It was observed that the dose of oxaliplatin in adjuvant over 5 cycles was positively associated with DFS (90% vs. 30%, P=0.011) in responders. The dose of oxaliplatin in adjuvant (greater than 5 cycles) was positively associated with OS (92% vs. 68%, P=0.018) in these patients. And finally, the dose of oxaliplatin in adjuvant (postoperative) in the range of 4–5 cycles was positively associated with metastasis-free survival in these responders (90% vs. 30%, P=0.003). This finding is consistent with observations in multicenter clinical trials in which patients with pCR after chemoradiotherapy had an excellent prognosis with little risk of developing distal metastases (22). Conversely, residual ganglion metastases after pre-operative radio-chemotherapy (especially ypN2) suggest a more aggressive tumor biology that is probably not very sensitive to any type of conventional chemotherapy (23). The FOLFOX regimen (oxaliplatin, 5-FU and leucovorin) followed in our study could have reduced the incidence of perioperative subclinical metastases, supporting the hypothesis that oxaliplatin could improve systemic control. However, the 5-year follow-up DFS data in the entire cohort exceeded 74% with a dose of oxaliplatin in adjuvant (postoperative) greater than 5 cycles compared to an equal or lower dose (74% vs. 48.8%, P=0.064), suggesting that the dose of oxaliplatin may have influenced on the decrease of distant metastases in our study, therefore, oxaliplatin should continue to be considered as a valuable agent to improve systemic control of micrometastasis. It should be emphasized that, in the context of an innovative clinical practice, the selection of high-risk patients (non-responders) was favored for adjuvant treatment.
To conclude, this work confirms that the responders to neoadjuvant with oxaliplatin, measured with highly reliable methodology (validated microscopic pathological response scales), defines a population of oxaliplatin-sensitive patients that benefit significantly from the administration of adjuvant oxaliplatin in cumulative enough doses (more than 5 cycles).
Although the methodology used is not robust from the experimental point of view, however, it allows to guide the care innovation on our own institutional experience (to treat intensively the disease in adjuvant for oxaliplatin-sensitive patients in neoadjuvant) and generate hypotheses for the design of care strategies (nomograms) and studies adapted to the risk of oncological heterogeneity.
Acknowledgements
We would like to thank the support offered by the Pharmacy Service of the General University Hospital Gregorio Marañón as well as all the Oncologists who have participated in the care and treatment of patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by Comité Ético del Hospital General Universitario Gregorio Marañon and informed consent was obtained from all patients.
References
- Lee M, Gibbs P, Wong R. Multidisciplinary Management of Locally Advanced Rectal Cancer--An Evolving Landscape? Clin Colorectal Cancer 2015;14:251-61. [Crossref] [PubMed]
- Havenga K, Enker WE, Norstein J, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol 1999;25:368-74. [Crossref] [PubMed]
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457-60. [Crossref] [PubMed]
- Resende HM, Jacob LF, Quinellato LV, et al. Combination chemotherapy versus single-agent chemotherapy during preoperative chemoradiation for resectable rectal cancer. Cochrane Database Syst Rev 2015;10.
- Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Glen H, Cassidy J. Redefining adjuvant chemotherapy in patients with stage III colon cancer: X-ACT trial. Expert Rev Anticancer Ther 2008;8:547-51. [Crossref] [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [Crossref] [PubMed]
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [Crossref] [PubMed]
- Calvo FA, Sole CV, Serrano J, et al. Preoperative chemoradiation with or without induction oxaliplatin plus 5-fluorouracil in locally advanced rectal cancer. Long-term outcome analysis. Strahlenther Onkol 2014;190:149-57. [Crossref] [PubMed]
- Huh JW, Kwon SY, Lee JH, et al. Comparison of restaging accuracy of repeat FDG-PET/CT with pelvic MRI after preoperative chemoradiation in patients with rectal cancer. J Cancer Res Clin Oncol 2015;141:353-9. [Crossref] [PubMed]
- Mak D, Joon DL, Chao M, et al. The use of PET in assessing tumor response after neoadjuvant chemoradiation for rectal cancer. Radiother Oncol 2010;97:205-11. [Crossref] [PubMed]
- Pomerri F, Pucciarelli S, Maretto I, et al. Prospective assessment of imaging after preoperative chemoradiotherapy for rectal cancer. Surgery 2011;149:56-64. [Crossref] [PubMed]
- Heijnen LA, Maas M, Beets-Tan RG, et al. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016;31:1157-62. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Rodel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]
- Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200-7. [Crossref] [PubMed]
- Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol 2014;113:223-9. [Crossref] [PubMed]
- Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356-62. [Crossref] [PubMed]
- Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696-701. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [Crossref] [PubMed]
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [Crossref] [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 2014;32:1554-62. [Crossref] [PubMed]


