Stereotactic body radiation therapy in primary hepatocellular carcinoma: current status and future directions
Introduction
Primary hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, which itself is the fifth-most common cancer worldwide and ranks third in cancer-related mortality (1). The standard management of primary HCC depends upon a number of factors including the patient’s tumor stage, baseline liver function, and performance status. Treatment options include surgery, chemotherapy, and radiation therapy (RT) (2). One recent advance in RT is stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR). In contrast to traditional RT, which involves small doses of radiation in daily treatments over the course of weeks, SBRT involves one to five treatments at relatively high biologically effective doses of radiation to treat tumors. The impetus for the development of this newer form of RT was technological advancements in immobilization, real-time imaging, and respiratory motion compensation, which have resulted in more precise targeting of pathologic structures while more effectively sparing the surrounding normal tissue (3).
SBRT finds its origins in stereotactic radiosurgery (SRS), a similar form of radiation treatment that was developed to treat intracranial tumors (4). Following encouraging results (5), it was expanded to extracranial tumors in the form of SBRT. Studies on SBRT have demonstrated good control rates and toxicity outcomes in the lung (6-8), prostate (9-11), and head and neck (12-14). One of the first studies of the use of SBRT in the treatment of primary HCC was done by Méndez Romero et al., which demonstrated the treatment modality’s potential in terms of LC and treatment toxicity (15). Since then, there has been a proliferation of both retrospective and prospective studies investigating the efficacy and toxicity profiles related to SBRT. In this review paper, we aim to summarize the relevant literature concerning the use of SBRT as a treatment modality for primary HCC, focusing on retrospective and prospective outcomes, as well as toxicity, comparisons of SBRT to alternative treatment modalities, and future directions. To do so, we carried out a literature search of articles accessible on PubMed through January 2018 related to the treatment of primary HCC with SBRT and now present our findings.
Prospective studies
The majority of prospective studies on SBRT in HCC patients were conducted on patients with Childs-Pugh Class A (CP-A) and Childs-Pugh Class B (CP-B). In aggregate, these studies provided evidence of the safety and efficacy of SBRT in treating HCC. Two-year local control (LC) ranged from 64% (16) to 95% (17). Overall survival (OS) rates ranged from 34% (2-year) (18) to 66.7% (3-year) (19). There was variation in the dosimetric parameters used in each trial (Table 1), with dosimetric plans ranging from 12 Gy in 3 fractions to 55 Gy in 5 fractions (20,21).

Full table
Phase I trials
One of the first phase I trials investigating SBRT in HCC patients was conducted by Méndez Romero et al. in 2006 and included 8 and 17 HCC and metastatic liver cancer patients, respectively. In the HCC subgroup, the number of patients with CP-A, CP-B, and no cirrhosis were 5, 2, and 1, respectively. The two most common dose fractionation schedules were 37.5 Gy in 3 fractions and 25 Gy in 5 fractions, with one patient receiving 30 Gy in 3 fractions. Two cases of RILD were reported, 1 classic and 1 non-classic. One CP-B HCC patient experienced Grade 5 toxicity (decompensated portal HTN, bleeding esophageal varices, and urinary tract infection, resulting in death), and the 1-year crude LC rate was 80% (15).
Phase II trials
Phase II trials to date have demonstrated promising LC and OS with minimal toxicities. Two recent phase II trials with relatively robust sample sizes will now be detailed.
Takeda et al. 2016 included 90 patients with HCC treated with SBRT of 40 Gy in 5 fractions (80 patients) or 35 Gy in 5 fractions (10 patients) and optional transarterial chemoembolization (TACE). The majority of patients (82/90) were CP-A, and the remainder were CP-B. Median OS was 54.7 months, with 3-year OS at 66.7%. An excellent 3-year LC rate of 96.3% was reported. Grade 3 toxicities included elevated transaminases and thrombocytopenia in 2 and 4 patients, respectively. There were no instances of grade 4 or grade 5 liver toxicities (19).
Feng et al. 2017 examined 69 patients with unresectable primary HCC along with 17 and 4 patients with metastatic liver disease and intrahepatic cholangiocarcinoma (IHC), respectively. Dose fractionation schedule was 45 Gy (median) in 5 fractions. In the HCC sub-group, 2-year LC and OS were 95%. One- and two-year OS rates in the HCC subgroup were 42% and 19%, respectively. Recurrence of HCC was observed in 3 patients. In the subset of 73 patients with primary liver tumors, toxicities included grade 2 ascites (1 patient), grade 3 ascites (1 patient), and grade 3 duodenal bleeding (1 patient) 7 months after completing treatment (22).
Retrospective studies
Retrospective studies have shown that SBRT for the treatment of localized, unresectable HCC is safe and effective (Table 2). Included in Table 2 are retrospective studies focusing specifically on SBRT in HCC with a sample size of 60 or more patients.
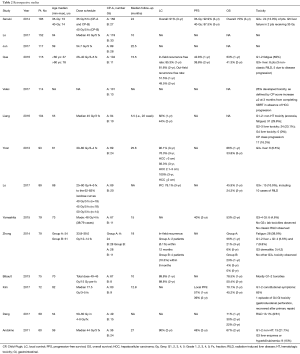
Full table
Numerous retrospective studies have found SBRT to be safe and effective in both small HCC (<5 cm) (23-28) and large HCC (>10 cm) (29-31). Two-year LC, progression-free survival, and OS rates ranged from 44–90% (32,33), 39–48% (24,34), and 24–67% (24,35), respectively. Some of this variability may be explained by variability of characteristics between study populations.
One of the largest retrospective studies on HCC patients receiving SBRT was published in 2014 by Sanuki et al. (26) and will now be examined in greater detail. One hundred and eighty-five patients with single, unresectable HCC lesion, measuring 5 cm or less, were included in this study. Patients with CP-A and CP-B were treated with 40 and 35 Gy in 5 fractions, respectively, between March 2005 and March 2012. The primary endpoints of 3-year LC and OS were 91% and 70% respectively. There was no statistically significant difference in the 3-year rates of LC (log-rank P=0.99) and OS (log-rank P=0.54) between the 35 and 40 Gy groups. Post-treatment causes of death included HCC progression, decompensated liver failure, and non-hepatic causes. The only toxicity reported during treatment was mild fatigue, experienced by 9 patients. Grade 3 laboratory abnormalities were observed in 6 patients prior to treatment. These improved to Grade 1–2 abnormalities during treatment. Two patients, both CP-B receiving 35 Gy treatments, experienced Grade 5 liver toxicity at 3 and 6 months post-treatment, respectively.
A more recent study was published in 2017 by Lo et al. that retrospectively analyzed 89 patients with HCC treated with SBRT. Notably, all patients included in this study had advanced HCC as defined by Barcelona clinic liver cancer (BCLC) stage C. The most common dose fractionation schedules were 40, 45, and 50 Gy in 5 fractions each (n=19, 18, and 14, respectively). Complete response and partial response were achieved in 22 (26.2%) and 42 (50.0%) patients. One- and three-year OS rates were 45.9% and 24.3%. These OS rates are relatively low compared to other studies, possibly due to the cohort’s advanced disease. The in-field control rate at 3 years was 78.1%. Extrahepatic spread, main portal vein thrombosis, and CP class (A vs. B) were found to be associated with OS. Toxicities included RILD in 10 patients (11.2%), of which 1 was classic RILD and 8 were non-classic RILD, and one met criteria for both types. Two patients went on to die from non-classic RILD (35).
Toxicity
Numerous retrospective studies have correlated acute liver toxicities with higher baseline CP scores (36-40), with other studies finding toxicity to be correlated with survival outcomes (26,27). Son et al. 2010 found that just 2/60 patients with baseline CP score of A5 experienced hepatic toxicity, compared with 4/10 and 2/4 patients with baseline CP of A6 and B7, respectively (38). Velec et al. reported liver toxicity in 26% of patients, as defined by an increase in CP score of ≥2 points within 3 months of completing SBRT. In a subgroup of 101 CP-A patients, a baseline score of A6 vs. A5 was associated with increased risk of liver toxicity (OR 4.85, P=0.0097). Other factors associated with increased toxicity in multivariate analysis included lower baseline platelet counts (OR 0.90, P=0.019), and several dose-volume parameters, including mean liver dose, effective volume, and dose to 700–900 cc of liver (37).
Studies focusing on SBRT’s role in hepatobiliary toxicity have provided variable results. In a cohort of 49 HCC patients and 1 patient with liver metastases with tumors adjacent to the central biliary system, treated by SBRT with >20 Gy, Eriguchi et al. reported just 1 patient who experienced significant radiation-induced bile duct stenosis (41). However, a study done in 2017 found that 7/40 HCC patients treated with SBRT experienced G3+ HB toxicities, and that the volume of central hepatobiliary tract irradiated to a biologically effective dose of 40 and 30 Gy with an alpha/beta ratio of 10, or VBED1040 (OR 1.049, P=0.0042) and VBED1030 (OR 1.052, P=0.0091), were associated with hepatobiliary toxicity (42).
The relationship between the change in laboratory values and clinical outcomes has been studied for HCC patients receiving SBRT. Serum alpha-fetoprotein (AFP) is commonly ordered in patients with HCC to monitor disease activity (citation needed). One retrospective study found that AFP normalization within 3 months of SBRT treatment was positively correlated with increased OS and PFS in the study population (43). Another study showed that the albumin-bilirubin score (ALBI) may be used to predict survival and post-treatment hepatic toxicity in this patient population (44)
RFA vs. SBRT
Recent studies have compared the efficacy of SBRT with radiofrequency ablation (RFA) in the treatment of HCC. RFA is one of the main non-surgical treatments of HCC (45) and has been shown to be effective in HCC <3 cm (46). Randomized controlled trials comparing RFA to SBRT are lacking, and two retrospective studies and one modeling study come to different conclusions regarding the treatment modalities’ relative efficacy. Nonetheless, these studies support the effectiveness of SBRT in treating small HCC. The three studies will now be discussed in further detail.
Wahl et al. 2016 reported on a cohort of 224 patients with inoperable, non-metastatic HCC treated with either SBRT (n=161) or RFA (n=63) from 2004 to 2012. One- and two-year FFLP was 83.6% and 80.2% in the RFA group, compared with 97.4% and 83.8% in the SBRT group. For tumors <2 cm, there was no statistically significant difference in FFLP between the two treatment groups. However, for tumors ≥2 cm, FFLP was superior in the SBRT group compared with the RFA group (HR, 3.35; 95% CI, 1.17 to 9.62, P=0.025). There was no statistically significant difference in OS between the two groups (1- and 2-year OS of 70% and 53% in the RFA group vs. 76% and 46% in the SBRT group) or in acute grade 3+ toxicities (11% and 5% with RFA and SBRT, respectively) (47). While this was a retrospective study, it suggests that SBRT may be superior to RFA in HCC tumors ≥2 cm.
Seo et al. 2016 provides more support for the use of SBRT in small HCC. Simulated outcomes for 20,000 virtual patients treated with either RFA or SBRT were analyzed. Two-way sensitivity analysis of their Markov model showed no difference between SBRT and RFA for tumors <2 cm in size. However, for tumors between 2–3 cm in size, SBRT yielded a lower 1-year local recurrence rate than RFA (1-year LR 0.2109 and 0.0541 in the SBRT and RFA groups, respectively) (48). These results suggest that SBRT is equally effective as RFA in the treatment of small HCC (<3 cm) and may be superior to RFA in the treatment of tumors ≥2 cm.
In contrast to the previous two studies, a National Cancer Database study of 3,980 patients with localized (i.e., stage I/II), non-surgically managed HCC, propensity-matched analysis and inverse probability-weighted analysis found a statistically significant survival advantage in patients treated with RFA versus SBRT [i.e., 5-year OS 29.8% (95% CI, 24.5% to 35.3%) vs. 19.3% (95% CI, 13.5% to 25.9%), P<0.001], even when accounting for the effects of fibrosis and cirrhosis (49).
Comparing toxicities associated with SBRT and RFA, Shiozawa et al. 2015 reported a higher adverse event rate in HCC patients treated with CyberKnife vs. RFA (11.4% vs. 0%). Additionally, the study found a statistically significant increase in Child-Pugh score in the SBRT subgroup, as well as a Child-Pugh score in the SBRT subgroup that was significantly higher than that of the RFA subgroup, 12 months after treatment (50).
Sorafenib vs. SBRT
Sorafenib is a tyrosine kinase inhibitor that is currently the standard of care in treating advanced HCC (51). The oral chemotherapy drug inhibits tumor cell proliferation and angiogenesis and increases the rate of apoptosis in tumor models by inhibiting vascular endothelial growth factor (VEGF) receptors (VEGFR-1, VEGFR-2, VEGFR-3), platelet-derived growth factor receptor- (PDGFR-) β, RET, c-KIT, FMS-like tyrosine kinase-3, and the Ras/MAPK pathway. There have been very few phase I clinical trials showing the effects of SBRT in combination with sorafenib in HCC patients (52-55). Brade et al. treated 16 patients with locally advanced Child-Pugh class A HCC who were ineligible for standard local-regional therapies with SBRT and concurrent sorafenib, which was given at 2 dose levels, 7 days before SBRT, and continued for 9 weeks after SBRT (53). No dose-limiting toxicities (DLTs) were observed in patients with an effective irradiated liver volume (Veff) <30% and sorafenib 400 mg daily, whereas worsening of Child-Pugh class was seen in 6 of 12 patients with Veff of 30–60% (30–33 Gy in 6 fractions). Additionally, 2 of 3 patients in the high Veff stratum treated with sorafenib 400 mg experienced gastrointestinal DLTs an average of 39 days after SBRT. Another phase I trial in patients with liver metastases found that 33% of patients experienced Grade 3+ toxicity at a median of 10 days, and OS was 22.3 months for those with effective liver volume irradiated (Veff) <80% (54). A study using the same radiation dose and sorafenib schedule found a median survival of 14 months and no DLTs (55). The increased toxicity of SBRT and sorafenib has also been found in patients with abdominal tumors undergoing SBRT, and studies have shown that there is a significant correlation between serious bowel injury and treatment with VEGFI therapy within 3 months of SBRT (P=0.0006) but not between bowel injury and radiation therapy bowel dose (P=0.20) (56). One hypothesis for this mechanism is that VEGF inhibitors may prevent normal tissue recovery in the post-SBRT period and thus result in greater SBRT-related toxicity (57).
A retrospective study looking at the results of previous studies found that in the pre-SBRT period (after 1 week of sorafenib), the median liver volume reduction observed was 68 cc (58). This effect was even more pronounced in focal tumor patients, who exhibited a median volume reduction of 98 cc. In addition, 47% of patients had reductions larger than the 95% intraobserver contouring error. The study did not find any significant changes in liver volume between planning and first SBRT in patients treated with SBRT alone. Statistically significant reduction in tumor perfusion has also been observed just 1 week after sorafenib administration (P<0.5) (59). These studies demonstrate that sorafenib may have an effect on normal liver, and careful reassessment of liver volume changes prior to SBRT may be necessary in patients.
TACE vs. SBRT
TACE is the treatment of choice for unresectable tumors that are too large or multifocal for other percutaneous ablation techniques such as RFA. TACE is also commonly used as a bridge for patients awaiting liver transplant, and is rarely effective completely when used alone. Thus, SBRT has been used in combination with TACE. In a phase 2 trial of HCC patients with inoperable tumors <10 cm, 38.3% of patients achieved complete remission within 6 months after completing SBRT. The 2-year LC rate, OS rate and progression-free survival rate were 94.6%, 68.7% and 33.8%, respectively. Additionally, 6.4% of patients experienced grade 3 gastrointestinal toxicity and 4.3% experienced grade 4 gastric ulcer perforation (60). In another study, which compared patients treated with TACE + SBRT to those who had only been treated with TACE, TACE+SBRT patients had significantly higher disease-free survival (DFS) rates. The mean DFS time in the SBRT group was 15.2 months, compared to 4.2 months in the TACE group (61). Two retrospective studies have also shown decreased local recurrence rates and higher OS rate in the combined TACE + SBRT groups compared to TACE only (33 vs. 20 months, respectively, P=0.02) (62,63). The rates of toxicity were also very low in both studies—between 2–7% of patients experienced grade 2–4 gastrointestinal toxicity, and in both studies one patient developed chest wall/rib pain after SBRT.
A study in 2013 found that the order in which the treatment was administered (SBRT then TACE versus TACE then SBRT) did not have any significant effect on response rate, survival rate, α-fetoprotein level restoration rate and rate of improvement of abdominal distention and discomfort (64). These rates were all significantly higher compared to the group that only received SBRT. However, the study found that the exacerbation rate of liver function (as measured by Child-Pugh grade) was lower in the group that received SBRT followed by TACE, compared to TACE followed by SBRT. There have not been any other studies that have attempted to administer SBRT followed by TACE. Further studies are needed to better understand this relationship.
Orthotopic liver transplantation (OLT)
Based on retrospective studies to date, SBRT is an emerging alternative to traditional bridges to OLT such as TACE, RFA, and radioembolization, in the treatment of unresectable HCC. A retrospective study of 209 HCC patients with 1–2 tumors undergoing SBRT (n=125) or TACE (n=84) prior to OLT found no difference in OS and better LC in the SBRT group compared to the TACE group (65). Another study compared SBRT with yttrium-90 radioembolization, RFA, or TACE prior to OLT and found a pathologic complete response to treatment in 28.5%, 41%, 60% and 75% of patients treated with TACE, SBRT, RFA, and Y90, respectively, with lower levels of acute toxicity in SBRT and Y90 (66). This was consistent with the 27.3% complete pathological response rate in a separate study of 16 patients receiving SBRT followed by OLT (67).
Despite encouraging pathologic response rates, their correlation to radiographic response has yet to be proven. In a retrospective study of 38 patients undergoing SBRT prior to OLT, radiographic response (68%) did not correspond to pathological response, as 74% of patients were found to have viable tumor post-resection. Additionally, a statistically significant positive correlation existed between initial tumor burden and treatment failure (68).
Discussion
In summary, SBRT has demonstrated promising results in the treatment of primary HCC. The current body of literature pertaining to SBRT in the treatment of HCC demonstrates the utility of SBRT in delivering clinical outcomes and toxicity rates comparable to those of more established radiation modalities, such as 3D-conformal radiotherapy (3D-CRT) and IMRT. Additionally, SBRT is well-tolerated and provides good survival and toxicity outcomes when compared to alternative treatment modalities, including TACE, RFA, and sorafenib. Several areas of research could play a significant role in further elucidating the clinical utility of SBRT in the treatment of HCC.
Volumetric modulated arc therapy (VMAT)
One of the disadvantages of SBRT using noncoplanar 3D-CRT is prolonged duration of each treatment session as compared to conventional radiation treatment schedules. Prolonged treatment times decrease patients’ ability to tolerate the procedure and predispose them to higher intra-treatment motion (69). VMAT has been studied in the treatment of HCC and has demonstrated the potential to reduce treatment times while fulfilling dose-constraint requirements (70,71).
Adaptive radiotherapy
One area of future inquiry relates to adaptive radiotherapy. Adaptive radiotherapy has been explored using various radiotherapy techniques as a means of selective dose escalation (72). However, the application of adaptive SBRT in HCC is not well studied. A recent phase 2 trial of patients with intrahepatic tumors and preexisting liver dysfunction treated with adaptive SBRT demonstrated high rates of LC at 2-year follow-up (17). SBRT doses were split into two stages, with a 4-week treatment gap in the middle during which the indocyanine green assay was used as a surrogate of liver function to identify patients at high risk of liver toxicity and adjust radiation doses accordingly. Further inquiry into both image-guided (73,74) and indocyanine green assay-based adaptive SBRT may hold promise for patients with poor baseline liver function who are at high risk of RILD.
Cost effectiveness
Few attempts have been made to evaluate the cost-effectiveness of SBRT compared to other HCC treatment modalities. A study comparing the cost-effectiveness of sorafenib and SBRT in advanced HCC in Taiwan found that SBRT was cost-effective at a willingness to pay threshold as defined by WHO guidelines (75). A Markov modeling study comparing a hypothetical cohort of inoperable, localized HCC patients treated with SBRT and RFA found that RFA may be more cost-effective in initial treatment, but SBRT may be more cost-effective for salvage therapy of local recurrences (76). Further cost analysis studies of SBRT in HCC can inform treatment planning in cases where SBRT and another treatment modality are equivalent in terms of clinical and toxicity outcomes.
Conclusions
SBRT is a newer form of radiation therapy that has been effectively applied in the treatment of primary HCC, demonstrating adequate survival and toxicity outcomes in both retrospective and prospective studies. Studies on SBRT’s role as an adjunct to TACE and sorafenib, a substitute for RFA, and a bridge to orthotopic liver transplant, have all demonstrated a potential role for SBRT, although additional prospective studies must confirm these findings. Future studies investigating SBRT’s cost-effectiveness and potential application in adaptive radiotherapy would provide valuable information regarding the clinical utilization of SBRT in primary HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533-43. Erratum in: Lancet Oncol 2001;2:596. [Crossref] [PubMed]
- Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20:4115-27. [Crossref] [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100-7. [Crossref] [PubMed]
- Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry 1983;46:797-803. [Crossref] [PubMed]
- Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol 2014;9:155. [Crossref] [PubMed]
- Woody NM, Stephans KL, Marwaha G, et al. Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors Greater Than 5 cm: Safety and Efficacy. Int J Radiat Oncol Biol Phys 2015;92:325-31. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [Crossref] [PubMed]
- Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013;8:58. [Crossref] [PubMed]
- Lischalk JW, Kaplan ID, Collins SP. Stereotactic Body Radiation Therapy for Localized Prostate Cancer. Cancer J 2016;22:307-13. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877-82. [Crossref] [PubMed]
- McDonald MW, Lawson J, Garg MK, et al. ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys 2011;80:1292-8. [Crossref] [PubMed]
- Lim CM, Clump DA, Heron DE, et al. Stereotactic Body Radiotherapy (SBRT) for primary and recurrent head and neck tumors. Oral Oncol 2013;49:401-6. [Crossref] [PubMed]
- Kodani N, Yamazaki H, Tsubokura T, et al. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res 2011;52:24-31. [Crossref] [PubMed]
- Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol 2006;45:831-7. [Crossref] [PubMed]
- Scorsetti M, Comito T, Cozzi L, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol 2015;141:1301-9. [Crossref] [PubMed]
- Feng M, Suresh K, Schipper MJ, et al. Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:40-7. [Crossref] [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [Crossref] [PubMed]
- Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016;122:2041-9. [Crossref] [PubMed]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [Crossref] [PubMed]
- Weiner AA, Olsen J, Ma D, et al. Stereotactic body radiotherapy for primary hepatic malignancies - Report of a phase I/II institutional study. Radiother Oncol 2016;121:79-85. [Crossref] [PubMed]
- Feng Z, Tao C, Zhu J, et al. An integrated strategy of biological and physical constraints in biological optimization for cervical carcinoma. Radiat Oncol 2017;12:64. [Crossref] [PubMed]
- Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 2010;10:475. [Crossref] [PubMed]
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. [Crossref] [PubMed]
- Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One 2013;8. [Crossref] [PubMed]
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399-404. [Crossref] [PubMed]
- Kimura T, Aikata H, Takahashi S, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res 2015;45:378-86. [Crossref] [PubMed]
- Su TS, Liang P, Liang J, et al. Long-Term Survival Analysis of Stereotactic Ablative Radiotherapy Versus Liver Resection for Small Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2017;98:639-46. [Crossref] [PubMed]
- Dang YZ, Li X, Huang SG, et al. Curative effect of stereotactic body radiotherapy for unresectable massive primary liver cancer. Mol Clin Oncol 2017;6:911-6. [Crossref] [PubMed]
- Que JY, Lin LC, Lin KL, et al. The efficacy of stereotactic body radiation therapy on huge hepatocellular carcinoma unsuitable for other local modalities. Radiat Oncol 2014;9:120. [Crossref] [PubMed]
- Gkika E, Schultheiss M, Bettinger D, et al. Excellent local control and tolerance profile after stereotactic body radiotherapy of advanced hepatocellular carcinoma. Radiat Oncol 2017;12:116. [Crossref] [PubMed]
- Liang P, Huang C, Liang SX, et al. Effect of CyberKnife stereotactic body radiation therapy for hepatocellular carcinoma on hepatic toxicity. Onco Targets Ther 2016;9:7169-75. [Crossref] [PubMed]
- Bibault JE, Dewas S, Vautravers-Dewas C, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One 2013;8. [Crossref] [PubMed]
- Que J, Kuo HT, Lin LC, et al. Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma. BMC Cancer 2016;16:451. [Crossref] [PubMed]
- Lo CH, Yang JF, Liu MY, et al. Survival and prognostic factors for patients with advanced hepatocellular carcinoma after stereotactic ablative radiotherapy. PLoS One 2017;12. [Crossref] [PubMed]
- Jun BG, Kim YD, Cheon GJ, et al. Clinical significance of radiation-induced liver disease after stereotactic body radiation therapy for hepatocellular carcinoma. Korean J Intern Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Velec M, Haddad CR, Craig T, et al. Predictors of Liver Toxicity Following Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2017;97:939-46. [Crossref] [PubMed]
- Son SH, Choi BO, Ryu MR, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys 2010;78:1073-80. [Crossref] [PubMed]
- Jung J, Yoon SM, Kim SY, et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 2013;8:249. [Crossref] [PubMed]
- Bae SH, Kim MS, Jang WI, et al. Low Hepatic Toxicity in Primary and Metastatic Liver Cancers after Stereotactic Ablative Radiotherapy Using 3 Fractions. J Korean Med Sci 2015;30:1055-61. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Acceptable toxicity after stereotactic body radiation therapy for liver tumors adjacent to the central biliary system. Int J Radiat Oncol Biol Phys 2013;85:1006-11. [Crossref] [PubMed]
- Toesca DA, Osmundson EC, Eyben RV, et al. Central liver toxicity after SBRT: An expanded analysis and predictive nomogram. Radiother Oncol 2017;122:130-6. [Crossref] [PubMed]
- Jung J, Yoon SM, Han S, et al. Alpha-fetoprotein normalization as a prognostic surrogate in small hepatocellular carcinoma after stereotactic body radiotherapy: a propensity score matching analysis. BMC Cancer 2015;15:987. [Crossref] [PubMed]
- Lo CH, Liu MY, Lee MS, et al. Comparison Between Child-Turcotte-Pugh and Albumin-Bilirubin Scores in Assessing the Prognosis of Hepatocellular Carcinoma After Stereotactic Ablative Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:145-52. [Crossref] [PubMed]
- Massarweh NN, Park JO, Farjah F, et al. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg 2010;210:441-8. [Crossref] [PubMed]
- Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-6. [Crossref] [PubMed]
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. [Crossref] [PubMed]
- Seo YS, Kim MS, Yoo HJ, et al. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: a Markov model-based analysis. Cancer Med 2016;5:3094-101. [Crossref] [PubMed]
- Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol 2018;36:600-8. [Crossref] [PubMed]
- Shiozawa K, Watanabe M, Ikehara T, et al. Comparison of percutaneous radiofrequency ablation and CyberKnife(®) for initial solitary hepatocellular carcinoma: A pilot study. World J Gastroenterol 2015;21:13490-9. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Dawson LA, Brade A, Cho C, et al. Phase I Study of Sorafenib and SBRT for Advanced Hepatocellular Carcinoma. Int J Radiat Oncol 2012;84:S10-1. [Crossref]
- Brade AM, Ng S, Brierley J, et al. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2016;94:580-7. [Crossref] [PubMed]
- Goody RB, Brade AM, Wang L, et al. Phase I trial of radiation therapy and sorafenib in unresectable liver metastases. Radiother Oncol 2017;123:234-9. [Crossref] [PubMed]
- Brade AM, Kim J, Brierley J, et al. Phase I study of sorafenib and whole-liver radiation therapy (WLRT) or stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2012;84:S11-2. [Crossref]
- Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys 2013;87:73-80. [Crossref] [PubMed]
- Pollom EL, Deng L, Pai RK, et al. Gastrointestinal Toxicities With Combined Antiangiogenic and Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;92:568-76. [Crossref] [PubMed]
- Swaminath A, Knox JJ, Brierley JD, et al. Changes in Liver Volume Observed Following Sorafenib and Liver Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;94:729-37. [Crossref] [PubMed]
- Coolens C, Driscoll B, Moseley J, et al. Feasibility of 4D perfusion CT imaging for the assessment of liver treatment response following SBRT and sorafenib. Adv Radiat Oncol 2016;1:194-203. [Crossref] [PubMed]
- Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012;118:5424-31. [Crossref] [PubMed]
- Honda Y, Kimura T, Aikata H, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol 2013;28:530-6. [Crossref] [PubMed]
- Jacob R, Turley F, Redden DT, et al. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of Cancer 2012 (Oxford) 2015;17:140-9.
- Yamashita H, Onishi H, Matsumoto Y, et al. Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat Oncol 2014;9:112. [Crossref] [PubMed]
- Kang J, Nie Q. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Mol Clin Oncol 2014;2:43-50. [Crossref] [PubMed]
- Sapir E, Tao Y, Schipper MJ, et al. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:122-30. [Crossref] [PubMed]
- Mohamed M, Katz AW, Tejani MA, et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol 2015;1:35-42. [Crossref] [PubMed]
- Moore A, Cohen-Naftaly M, Tobar A, et al. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable Hepatocellular carcinoma. Radiat Oncol 2017;12:163. [Crossref] [PubMed]
- Mannina EM, Cardenes HR, Lasley FD, et al. Role of Stereotactic Body Radiation Therapy Before Orthotopic Liver Transplantation: Retrospective Evaluation of Pathologic Response and Outcomes. Int J Radiat Oncol Biol Phys 2017;97:931-8. [Crossref] [PubMed]
- Langen KM, Willoughby TR, Meeks SL, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys 2008;71:1084-90. [Crossref] [PubMed]
- Herbert C, Kwa W, Nakano S, et al. Stereotactic body radiotherapy: volumetric modulated arc therapy versus 3D non-coplanar conformal radiotherapy for the treatment of early stage lung cancer. Technol Cancer Res Treat 2013;12:511-6. [Crossref] [PubMed]
- Wang PM, Hsu WC, Chung NN, et al. Feasibility of stereotactic body radiation therapy with volumetric modulated arc therapy and high intensity photon beams for hepatocellular carcinoma patients. Radiat Oncol 2014;9:18. [Crossref] [PubMed]
- Yan D, Vicini F, Wong J, et al. Adaptive radiation therapy. Phys Med Biol 1997;42:123-32. [Crossref] [PubMed]
- Park SS, Yan D, McGrath S, et al. Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: clinical evidence. Int J Radiat Oncol Biol Phys 2012;83:947-52. [Crossref] [PubMed]
- Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer--dosimetric results from a prospective clinical trial. Radiother Oncol 2013;106:80-4. [Crossref] [PubMed]
- Leung HW, Liu CF, Chan AL. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol 2016;11:69. [Crossref] [PubMed]
- Pollom EL, Lee K, Durkee BY, et al. Cost-effectiveness of Stereotactic Body Radiation Therapy versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Markov Modeling Study. Radiology 2017;283:460-8. [Crossref] [PubMed]


