Role of lymph node ratio in selection of adjuvant treatment (chemotherapy vs. chemoradiation) in patients with resected gastric cancer
Introduction
Although surgery remains the mainstay of treatment for gastric cancer (GC) (1,2), a multimodal treatment approach is often required to extend survival due to a high incidence of locoregional recurrence and distant metastases after curative resection. Perioperative chemotherapy (PC) (3), adjuvant chemoradiation (CRT) (4), and adjuvant chemotherapy (CT) (5,6) have all been shown to improve survival when compared to surgery alone in randomized clinical trials. In the United States (U.S.), the Southwestern Oncology Group Intergroup trial (INT0116) was the first to show superior overall survival (OS) with adjuvant CRT compared to surgery-only among patients with resected GC [mortality hazard ratio (HR) of 0.74; 95% confidence interval (CI), 0.60–92] (4). However, this study has been heavily criticized for the surgical quality, as 54% of patients underwent D0 lymphadenectomy and only 10% had a D2 lymphadenectomy. This has limited the acceptance of adjuvant CRT outside of the U.S. with some critics interpreting the reported adjuvant CRT effect as merely compensating for inadequate surgery. Furthermore, this concern has prevailed despite results from retrospective studies that have confirmed improved survival for adjuvant CRT compared to surgery-only (7,8), even among patients who received a D2 lymphadenectomy (8).
To date, the South Korean Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) trial is the only clinical trial that has compared adjuvant CRT to CT among GC patients treated with D2 lymphadenectomy (9). Initial results from the ARTIST trial showed no significant difference in disease free survival (DFS) for patients treated with adjuvant CRT compared to adjuvant CT, with a 3-year DFS of 78.2% versus 74.2%, P=0.086, respectively. However, a subgroup analysis among subjects with lymph node (LN) positive disease showed superior DFS for adjuvant CRT compared to adjuvant CT [HR 0.69 (95% CI, 0.474–0.995), P=0.047] (9). Follow-up analyses of the ARTIST trial data showed that superior survival with adjuvant CRT was limited to GC patients with a lymph node ratio (LNR) greater than 25% (10). These findings, however, have never been evaluated in a Western population.
The objective of this study was to use prospectively collected California Cancer Registry (CCR) data to compare survival, stratified by LNR, among resected GC patients treated with either adjuvant CRT or adjuvant CT in a Western population. This comparison has not been made in randomized clinical trials in Western populations.
Methods
Study population
The CCR is a California statewide cancer surveillance program that has been operational since 1988 and maintains data on cancer occurrence, treatment and survival (11). Using CCR data and the International Classification of Disease for Oncology, Third Edition (12) (ICD-O-3) topographic codes C160-C169, records for patients diagnosed with gastric and gastroesophageal junction (GEJ) from 2004 through 2013 were retrieved. Additionally, ICD-O-3 morphology codes M-8120-M-8240 and M-8255-M-8576 were used to select patients diagnosed with adenocarcinoma, while the American Joint Committee on Cancer Staging, 6th Edition (AJCC 6) (13) rules were used to retain stage IB–III GC cases. Patients were classified into the CT cohort if chemotherapy was initiated after surgery with no receipt of pre- or postoperative radiotherapy. Similarly, patients were selected into the CRT group if chemoradiation was initiated following surgery. This study was conducted using existing data without patient contact and was approved by Loma Linda University Institutional Review Board [59061].
Study outcomes
The primary endpoint was OS, while gastric cancer-specific survival (GCSS) was a secondary endpoint. Survival was calculated as the time from surgery to death or the last date of study follow-up (December 31, 2014), whichever came first.
Study covariates
Demographic variables included in this study were age at diagnosis, sex and race/ethnicity (Asian/other, Hispanic, non-Hispanic black, non-Hispanic white). Additionally, tumor characteristics that included T-stage (13), histology type as intestinal (M-8144), diffuse (M-8145) or signet ring histology (M-8490) (12), tumor location (proximal/distal) and cardia/GEJ location (yes/no) were included in analyses as appropriate.
Statistical analyses
Demographic and tumor characteristics were summarized using counts and percentages with comparison between treatment cohorts conducted using χ2 tests. Median follow-up time was estimated using the inverse Kaplan-Meier curve (14), while Kaplan-Meier survival curves with log-rank tests were used to compare median survival times. To harmonize differences in demographic and tumor characteristics observed between adjuvant CRT and adjuvant CT treatment groups, propensity scores were used in a two-step process (15). First, demographic and tumor characteristics were used in a logistic regression model to predict treatment propensity scores. In a second step, propensity score (inverse probability weighting) weighted Cox proportional hazards models were used to estimated mortality hazards ratios (HR) with 95% CI limits. Log-log plots and Schoenfeld residuals were used to assess the proportionality assumption. All tests were two-sided, performed using a significance level of five percent (α=0.05) and conducted using SAS software, Version (9.4) of the SAS System for Windows. Copyright ©2002-2012 SAS Institute Inc.
Subgroup analyses
To evaluate the impact of positive LN disease burden on survival findings, all analyses were conducted within LNR strata. LNR is calculated as the number of positive LNs divided by the total count of all LNs examined (positive LNs/total count of nodes examined). In this study four LNR strata that include 0%, 1–9%, 10–25% and >26% as described by Marchet et al. (16) and adopted by the ARTIST trial were used (10). Furthermore, since dissection of at least 15 LNs is recommended for adequate staging and survival (1), separate analyses were conducted for patients that had less than 15 LNs and those with 15 or more LNs examined.
Results
Study population
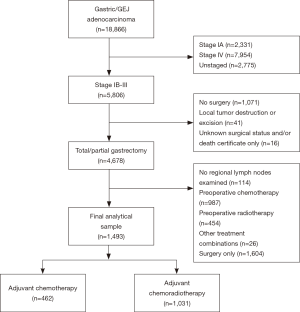
Between 2004 and 2013, 18,866 patients diagnosed with gastric/GEJ adenocarcinomas were identified, including 5,806 that were stage IB-III. Among these, 4,678 patients had been treated with total or partial gastrectomy. After excluding patients who did not receive any adjuvant treatment, underwent preoperative CT or CRT, and those who had no regional LNs examined, we derived a final analytical sample of 462 patients treated with CT and 1,031 that received CRT (Figure 1). Median follow-up was 76 months.
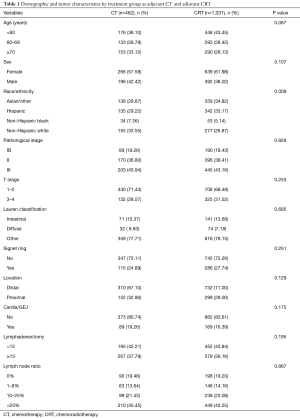
Patient’s age, sex, and tumor characteristics that include stage, histology type, and primary tumor location were comparable between the CT and the CRT cohort (Table 1). Additionally, there were no differences in the extent of LN dissection (P=0.12) and the distribution of LNR categories (P=0.87) between the two treatment cohorts (Table 1). Of note, nearly 60% of the patients had 15 or more LN evaluated. There were ethnic differences in the type of adjuvant treatment received. Non-Hispanic whites more likely to be treated with CT (33.6% vs. 26.9%), while Asian/other (29.9% vs. 34.8%) and Hispanic (29.2% vs. 33.2%) were more likely to have received CRT respectively (P=0.009).
Full table
CRT versus CT
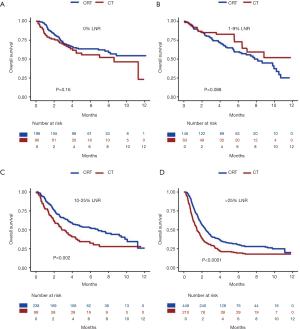
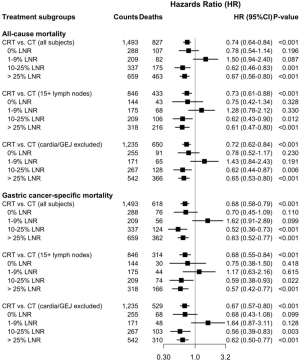
The median survival was 54 months for the CRT cohort versus 35 months for the CT cohort, P<0.001. Compared to CT, CRT was associated with longer median survival among patients with 10–25% LNR (P=0.002) and those with >25% LNR (P<0.0001) (Figure 2). No significant difference in median survival was observed between CRT and CT among patients with 0% LNR (P=0.16) and those with 1–9% LNR (P=0.088) (Figure 2). Further propensity score weighted analyses were performed for OS and GCSS for all patients, and for the subgroup of patients with ≥15 LN examined, and excluding patients with cardia/GEJ cancers. Among all patients, CRT was associated with improved survival compared to CT [HR 0.74 (95% CI, 0.64–0.84)]. This survival benefit was primarily seen among patients that had 10–25% LNR [HR 0.62 (95% CI, 0.46–0.83)] and those with >25% LNR [HR 0.67 (95% CI, 0.56–0.80)] (Figure 3). Comparable findings were observed for analyses that evaluated GCSS [HR 0.68 (95% CI, 0.58–0.79)]. Subgroup analysis was performed for patients with ≥15 LN evaluated to assess the outcome differences between treatment groups among patients who had adequate lymphadenectomy. Similar to the results observed for the entire study cohort, CRT was associated with improved all cause [HR 0.73 (95% CI, 0.61–0.88)] and GC specific mortality [HR 0.68 (95% CI, 0.55–0.84)] (Figure 3). As noted previously, this survival benefit was primarily observed among patients with ≥10% LNR. Due to the current treatment recommendations for preoperative chemoradiation in patients with GEJ cancer, we chose to perform a subgroup analysis excluding patients with cardia/GEJ tumors. The survival differences observed for this subgroup of patients were similar to that seen in the overall study population and the subgroup of patients with ≥15 LNs evaluated (Figure 3).
Discussion
Our study clearly demonstrates that adjuvant CRT is associated with significantly improved survival compared to adjuvant CT in patients with resected GC who have high metastatic LN disease burden (≥10% LNR) (Figure 2). The results of our study have important therapeutic implications for GC management. The benefit of adjuvant CRT compared to adjuvant CT in patients with GC after D2 gastric resection has been questioned after the publication of the ARTIST trial results (9). The final results of this trial, published after a median follow-up of 7 years showed no significant survival benefit between patients treated with CRT vs. CT after D2 gastric resection. However, based on a post-hoc subgroup analyses, the authors reported that adjuvant chemoradiotherapy might be of benefit in patients with LN positive disease, specifically those with LNR of ≥25%. Based on this finding, the ARTSIT II trial has been designed to evaluate the benefit of CRT compared to CT in patients with LN positive GC. The results of the ARTIST II trial are not expected to be available for few years. In the interim, it is important to assess the impact of LNR on the survival effects of CRT versus CT among patients treated with curative intent in a large Western population. To this end, we conducted the current study to aid clinicians in identifying patients who may benefit from CRT.
The importance of adequate LN assessment in GC has been well documented by several investigators (17). Although, the actual number of LN evaluated is important, the LNR has been shown to be more important in various cancers (18-20). Marchet et al. (16) reported the 5-year survival outcomes of patients who underwent adequate and inadequate lymphadenectomy, stratified based on the LNR categories (N0—0%, N1—1–9%, N2—10–25%, and N3—>25%). LNR ratio retained its significance as an independent prognostic factor, even among the patients who underwent inadequate lymphadenectomy. We utilized the methodology of Marchet et al. for LNR categorization in the current study and found that patients with ≥10% LNR have an associated survival advantage when treated with CRT compared to CT.
While we recognize that the ARTIST trial was a randomized trial, our results have important differences from that of the ARTIST trial. First, we found that CRT was associated with improved survival compared to CT among all patients, which is in contrast to the results of ARTIST trial which showed no survival differences between CRT and CT (9). This is likely due to a higher proportion of patients with LNR >25%, thus higher statistical power, in our study for both CRT (45.45%) and CT (43.55%) compared to the ARTIST trial (19.87%) (9). Second, while in the current study, CRT was associated with improved survival among patients having LNR ≥10%, the survival benefit with CRT in the ARTIST trial was limited to subjects with a LNR greater than 25% (10). This difference could possibly be due to differences in the number of LN evaluated. In the ARTIST trial, the median number of LN evaluated were 40 [13–142] for CRT and 40 [12–84] for CT while the patients in the current study had a median of 16 [1–98] LN for CRT and 17 [1–98] for CT among all subjects, and 21 [15–90] LN for CRT and 23 [15–90] for CT among patients that had at least 15 LN examined (9). These differences highlight the importance of evaluating the role of CRT compared to CT in a Western population.
While CRT is often considered as a treatment modality for reduction of local and regional recurrence among patients that received an inadequate LN dissection (4,21), we observed that the survival benefit conferred by CRT in patients with ≥10% LNR persisted even after excluding patients who underwent inadequate LN dissection. This finding potentially addresses one of the major criticisms of the INT0116 trial. In the current study, anatomic sub-sites were identified using ICD-O-3 topographic codes that combine cardia and GEJ cases (C160). Since preoperative CRT is effective (22) and recommended (23) for GEJ tumors, we performed a subgroup analyses excluding patients with cardia/GEJ tumors. Exclusion of these patients did not significantly alter our results, indicating that our findings were not mainly dependent on the benefit of CRT in patients with GEJ cancers.
Several ongoing clinical trials are evaluating the role and timing of radiotherapy in patients with GC. Initial results of the CRITICS trial, designed to compare adjuvant CRT versus adjuvant CT among patients that received preoperative chemotherapy (24) were recently presented at the American Society of Clinical Oncology annual meeting. After a median follow-up of 50 months, the primary endpoint of 5-year survival was 41.3% for CT and 40.9% for CRT after receipt of neoadjuvant chemotherapy and adequate surgery (P=0.99) (25). The effect of LNR in this population however has not yet been examined (24,26). Other trials include the TOPGEAR trial which is assessing the effect of preoperative chemoradiotherapy compared to PC (23). The ARTIST II and CRITICS II trials are currently ongoing, as follow-up studies of the original trials among node positive patients.
Our study has significant implications to current clinical practice especially since adjuvant CRT and CT have not been compared in a clinical trial setting among Western patients who undergo upfront surgical resection. In addition, with the use of registry data containing a large number of patients with diverse demographics and well-matched cohorts, our findings are reflective of “real-world” practice in the West.
Limitations
Our study has several limitations that are inherent to large, population-based retrospective studies. Patient comorbidities and complications of treatment are not documented in the CCR. By design, only patients that underwent total or partial gastrectomy and started adjuvant treatment were included in this study allowing for increased homogeneity between the study cohorts and to minimize the potential effects of selection bias. Results from the ARTIST trial, suggested that CRT may reduce the rate of locoregional recurrence compared to CT (7% vs. 13% P=0.0033) (27). Information about cancer recurrence is not available within the CCR for such a comparison and hence disease-free survival could not be reported. Additionally, information about chemotherapeutic agents and number of chemotherapy cycles administered was also not available. Hence, outcomes were analyzed based on receipt of treatment. Early discontinuation of assigned treatment could have certainly impacted the outcomes and could not be accounted for.
Conclusions
Our study shows that LNR is an important and readily available tool that can aid adjuvant treatment decisions in patients with resected GC. CRT was associated with survival benefit compared to CT in patients with high LN disease burden (≥10% LNR) even after adequate lymphadenectomy with gastric resection. These results should be taken into consideration when offering adjuvant treatment for patients with LN positive disease. In addition, future clinical trials should take into account this stratification when assigning treatments. In the near future, genotypic classification of GC will likely guide treatment decisions. Until then, available tumor phenotypic characteristics should be used to the fullest extent to best select patients for various treatment options.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted using existing data without patient contact and was approved by Loma Linda University Institutional Review Board [59061].
References
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant Chemotherapy for Gastric Cancer with S-1, an Oral Fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Seyedin S, Wang PC, Zhang Q, et al. Benefit of Adjuvant Chemoradiotherapy for Gastric Adenocarcinoma: A SEER Population Analysis. Gastrointest Cancer Res 2014;7:82-90. [PubMed]
- Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005;63:1279-85. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Kim Y, Park SH, Kim KM, et al. The Influence of Metastatic Lymph Node Ratio on the Treatment Outcomes in the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) Trial: A Phase III Trial. J Gastric Cancer 2016;16:105-10. [Crossref] [PubMed]
- California Department of Public Health. California Cancer Registry, California Department of Public Health. 2014. Available online: http://www.ccrcal.org/index.shtml
- Fritz AG. International classification of diseases for oncology: ICD-O-3. Third edition. Available online: http://www.who.int/classifications/icd/adaptations/oncology/en/
- Greene FL, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 6th edition. New York: Springer, 2002.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]
- Marchet A, Mocellin S, Ambrosi A, et al. The Ratio Between Metastatic and Examined Lymph Nodes (N Ratio) Is an Independent Prognostic Factor in Gastric Cancer Regardless of the Type of Lymphadenectomy: Results From an Italian Multicentric Study in 1853 Patients. Ann Surg 2007;245:543-52. [Crossref] [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114-24. [Crossref] [PubMed]
- Zhang MR, Xie TH, Chi JL, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget 2016;7:72898-907. [PubMed]
- Costa WL Junior, Coimbra FJ, Batista TP, et al. Evaluation of N-ratio in selecting patients for adjuvant chemoradiotherapy after d2-gastrectomy. Arq Gastroenterol 2013;50:257-63. [Crossref] [PubMed]
- Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol 2006;24:2910-6. [Crossref] [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430-6. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers. J Natl Compr Canc Netw 2011;9:830-87. [Crossref] [PubMed]
- Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329. [Crossref] [PubMed]
- Verheij M, Jansen EP, Cats A, et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. J Clin Oncol 2016;34:4000.
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]