Anastomotic leak and neoadjuvant chemoradiotherapy in esophageal cancer
Introduction
It is estimated that there will be 16,910 new cases of esophageal cancer diagnosed, with 15,690 dying from the disease in the United States in 2016 (1). The majority of esophageal cancers are either adenocarcinoma or squamous cell carcinoma. Trimodality therapy of neoadjuvant chemoradiation (NCR) followed by surgical resection has been established as the standard of care for advanced disease (2,3). However, neoadjuvant therapy has been associated with significant perioperative morbidity and mortality (4).
Surgical resection continues to remain the preferred modality for locoregional esophageal cancer and improved perioperative care and advanced surgical techniques have contributed to reduced postoperative mortality. However, despite these advances, esophagectomy continues to be associated with significant morbidity and mortality (5,6). One of the most dreaded complications is anastomotic leak (AL). The incidence of AL after esophagectomy has been reported to range from 5–40% and leak associated mortality from 2–12% (7-19). The presence of AL can result in the need for reoperation, endoscopic stenting, nasogastric tube placement, percutaneous drainage, need for broad spectrum antibiotics, and significant stricture requiring multiple endoscopic dilations (15,20-22).
Several factors have been identified that are associated with increased AL including anastomotic technique, location of anastomosis, type of conduit, comorbid conditions, and neoadjuvant therapy (9,12,23,24). However, the association between NCR and AL has been questioned (3,25-30). The purpose of this study was to compare AL rates of upfront surgery vs. NCR.
Methods
Patients
A prospectively maintained and institutional review board approved esophagectomy database of more than 800 patients who underwent upfront surgery of surgery after NCR was queried to determine factors related to AL. Patients underwent surgery between 1996 and 2015. AL was compared between upfront surgery and NCR patients.
NCR
Patients were discussed in a weekly multi-disciplinary tumor board conference and pathology was reviewed at our institution. Pre-operative staging including endoscopic ultrasound, CT chest, abdomen and pelvis and PET scans. Patients who had locally advanced disease (≥T2 and/or ≥N1) were treated with NCR. The choice of chemotherapy was left to the discretion of the treatment medical oncologist. Patients received infusional 5-fluorouracil (5-FU) and cisplatin or weekly carboplatin and Taxol concurrent external beam radiation for a total dose of 45–59.4 Gy over the course of 5–7 weeks.
Surgery
Six weeks after the conclusion of therapy, patients underwent restaging with PET-CT scans. Patients without evidence of metastatic disease and good performance status were then offered open, laparoscopic or robotic esophagectomy at the discretion of the surgeon. Esophagectomy was performed during the 6–10-week window after conclusion of NCR. Patients are referred to cardiac and pulmonary specialists for pre-operative risk assessment and optimization prior to surgery.
Statistics
The primary endpoint was incidence of AL. Baseline univariate comparisons of patient characteristics between the upfront surgery patients and the NCR patients was made for continuous variables using both the Mann-Whitney U and Kruskal Wallis tests as appropriate. Pearson’s Chi-square test was used to compare categorical variables. Propensity score matching (PSM) was performed against a number of variables associated with AL. Multivariable Cox proportional hazard models were developed to identify predictors of AL included in the models were age, sex, tumor location, surgical technique, NCR, BMI, diabetes, and year of surgery. All statistical tests were two-sided and α (type I) error <0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 23.0 (IBM®, Chicago, IL, USA). This study was approved as exempt by the Institutional Review Board.
Results
There were 820 patients (288 UFS, 532 NCR) in the database that were identified. NCR patients were significantly younger, had increased use after 2010, were more likely to undergo robotic surgery, and were less likely to be obese. After PSM, there were 518 patients (259 UFS, 259 NCR) and the only difference between NCR and US patients was younger age (65 vs. 68; P=0.002) (Table 1).

Full table
Overall AL rate was 5.5% before PSM and 6% after PSM. Univariate analysis of factors related to differences in leak rates between upfront and neoadjuvant patients are presented in Table 2. Overall AL rates for UFS patients before and after PSM was 8.0% and 7.7% compared to 4.2% and 4.1% for NCR patients. Before PSM, there was a significantly decreased AL rate in NCR patients, however, this was not significant after PSM. There was a significantly decreased AL in NCR patients who had adenocarcinoma, underwent robotic Ivor-Lewis, were non-diabetic, and had normal BMI compared to UFS patients before PSM. After PSM, decreased AL in NCR patients was seen in non-diabetics and patients with normal BMI.
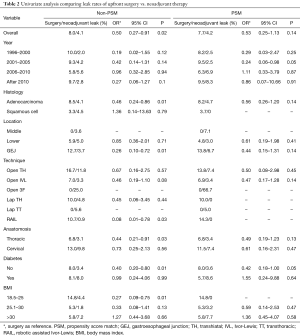
Full table
Table 3 displays multivariate analysis (MVA) of factors before and after PSM associated with AL in upfront surgery and NCR patients. MVA of factors associated with decreased AL in US patients were tumors of the lower esophagus and BMI >25, and confirmed on PSM. Age, gender, year of surgery, histology, anastomotic location, and diabetes were not predictive of AL in upfront surgery patients. In NCR patients, significantly decreased AL was observed in patient undergoing thoracic anastomosis. However, after PSM, no factors were prognostic for AL in NCR patients.
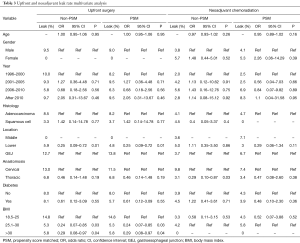
Full table
Table 4 displays MVA before and after PSM of factors associated with AL in all patients. Decreased AL was observed in NCR patients, patients with distal esophageal patients, thoracic anastomosis, and in patients with BMI from 25–30. However, after PSM, only distal esophageal tumors and thoracic anastomosis were prognostic of decreased AL.
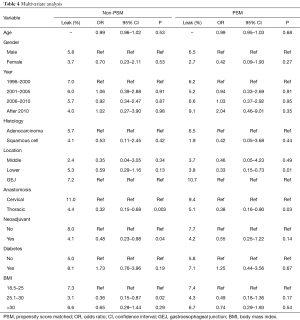
Full table
Discussion
AL is a dreaded complication of esophagectomy associated with significant morbidity and mortality. There is a wide range of incidence of AL as high as 40% with reported mortality associated with leak as high as 12% (7-19). The success of esophageal anastomosis depends not only on surgical technical factors but also patient related factors. Technical factors include location of anastomosis, type of conduit, and how the conduit is dissected and mobilized to ensure the formation of a tension-free anastomosis and maintaining a healthy blood supply to the conduit (9,12,23,24). Patient related factors include nutritional status, comorbid conditions, BMI, and NCR (9,12).
Overall AL was 5.4%. While we observed an overall decreased AL rate in NCR patients compared to US patients, after PSM the difference was not statistically significant. Factors correlating with decreased AL in UFS patients are distal tumors and BMI >25. While thoracic anastomosis correlated with decreased AL in NCR patients, the difference wasn’t significant after PSM. The only 2 factors we identified that were prognostic for decreased AL in all patients were distal tumors and thoracic anastomosis.
Decreased AL in NCR patients was restricted to patients undergoing a thoracic anastomosis but not in patients with cervical anastomosis. Very few studies have addressed the role of NCR on incidence of AL. In the CROSS trial, where most patients underwent a transhiatal technique with cervical anastomosis, there was a nonsignificant decrease in AL in patients who received NCR (22% vs. 30%) (3). However, Briel et al. reported an analysis of 393 patients with cervical anastomosis and demonstrated an increased incidence of AL in patients who received neoadjuvant therapy (9). This was hypothesized to be due to an increase in conduit ischemia which increased AL. However, cervical anastomosis requires the formation of a longer conduit and traverses a greater distance in the mediastinum, placing it under tension and potentially compromising the vascularity. Additionally, anatomic variations in the anatomy of the gastroepiploic artery may play a significant role, particularly in patients undergoing transhiatal esophagectomy. An analysis of >7,500 esophagectomy patients from the Society of Thoracic Surgeons Thoracic Database revealed that NCR was not associated with AL (12). Table 5 lists the most recent studies comparing AL between UFS and NCR patients. AL rates of UFS patient and NCR patients ranged between 0–30% and 2.9–14.8%, respectively, with no statistically significant differences (3,25-30).
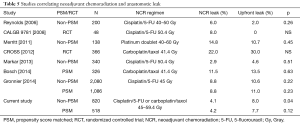
Full table
Several studies have confirmed comorbid conditions like diabetes correlate with AL but not BMI (31-33). Briel et al. reported that comorbid conditions requiring treatment strongly correlated with increased AL (9). In addition, while they demonstrated that an increased BMI correlated with stricture, it did not correlate with AL. Kayani et al. reported that diabetes in obese patients correlated with AL, but not in obese patients without diabetes (34). In an analysis of >7,500 esophagectomy patients from the Society of Thoracic Surgeons Thoracic Database revealed that diabetes and BMI >35 correlated with increased AL (12). It wasn’t reported but it is likely possible that the obese patients also were diabetic. The presence of comorbid conditions is thought to cause conduit ischemia resulting in AL. We found that non-diabetic patients undergoing NCR had lower AL compared to UFS on UVA but it was not significant on MVA and overall, we found that diabetes did not correlate with AL. On UVA, NCR patients with normal BMI had lower AL compared to UFS. In addition, we found that BMI >25–30 was associated with decreased AL in UFS patients but not in NCR patients. The additional fat/omentum in heavier patients may compress the conduit in the confined space of the posterior mediastinum and serve as a protective flap in overweight patients.
Conclusions
AL is a dreaded complication related to both technical and patient related factors. There is no association between NCR and AL in esophagectomy patients. Decreased AL is observed in US patients with distal tumors and BMI >25, while no factors correlated with AL in NCR patients. Distal tumors and thoracic anastomosis correlate with decreased AL.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by IRB Sarasota Memorial Hospital # GI-ONC.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Coupland VH, Lagergren J, Luchtenborg M, et al. Hospital volume, proportion resected and mortality from oesophageal and gastric cancer: a population-based study in England, 2004-2008. Gut 2013;62:961-6. [Crossref] [PubMed]
- Hanna GB, Boshier PR, Knaggs A, et al. Improving outcomes after gastroesophageal cancer resection: can Japanese results be reproduced in Western centers? Arch Surg 2012;147:738-45. [Crossref] [PubMed]
- Beitler AL, Urschel JD. Comparison of stapled and hand-sewn esophagogastric anastomoses. Am J Surg 1998;175:337-40. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41; discussion 541-2. [Crossref] [PubMed]
- Collard JM, Romagnoli R, Goncette L, et al. Terminalized semimechanical side-to-side suture technique for cervical esophagogastrostomy. Ann Thorac Surg 1998;65:814-7. [Crossref] [PubMed]
- Hsu HH, Chen JS, Huang PM, et al. Comparison of manual and mechanical cervical esophagogastric anastomosis after esophageal resection for squamous cell carcinoma: a prospective randomized controlled trial. Eur J Cardiothorac Surg 2004;25:1097-101. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Kim RH, Takabe K. Methods of esophagogastric anastomoses following esophagectomy for cancer: A systematic review. J Surg Oncol 2010;101:527-33. [Crossref] [PubMed]
- Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997;226:169-73. [Crossref] [PubMed]
- Martin LW, Hofstetter W, Swisher SG, et al. Management of intrathoracic leaks following esophagectomy. Adv Surg 2006;40:173-90. [Crossref] [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [Crossref] [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [Crossref] [PubMed]
- Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012;16:1287-95. [Crossref] [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [Crossref] [PubMed]
- Baba M, Aikou T, Natsugoe S, et al. Appraisal of ten-year survival following esophagectomy for carcinoma of the esophagus with emphasis on quality of life. World J Surg 1997;21:282-5; discussion 286. [Crossref] [PubMed]
- Licht E, Markowitz AJ, Bains MS, et al. Endoscopic Management of Esophageal Anastomotic Leaks After Surgery for Malignant Disease. Ann Thorac Surg 2016;101:301-4. [Crossref] [PubMed]
- Schaheen L, Blackmon SH, Nason KS. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 2014;208:536-43. [Crossref] [PubMed]
- Blackmon SH, Correa AM, Wynn B, et al. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007;83:1805-13; discussion 1813.
- Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. [Crossref] [PubMed]
- Bosch DJ, Muijs CT, Mul VE, et al. Impact of neoadjuvant chemoradiotherapy on postoperative course after curative-intent transthoracic esophagectomy in esophageal cancer patients. Ann Surg Oncol 2014;21:605-11. [Crossref] [PubMed]
- Gronnier C, Trechot B, Duhamel A, et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014;260:764-70; discussion 770-1. [Crossref] [PubMed]
- Markar SR, Bodnar A, Rosales J, et al. The impact of neoadjuvant chemoradiotherapy on perioperative outcomes, tumor pathology, and survival in clinical stage II and III esophageal cancer. Ann Surg Oncol 2013;20:3935-41. [Crossref] [PubMed]
- Merritt RE, Whyte RI, D'Arcy NT, et al. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. Ann Thorac Surg 2011;92:2034-40. [Crossref] [PubMed]
- Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg 2006;132:549-55. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Melis M, Weber J, Shridhar R, et al. Body mass index and perioperative complications after oesophagectomy for adenocarcinoma: a systematic database review. BMJ Open 2013;3. [Crossref] [PubMed]
- Salem AI, Thau MR, Strom TJ, et al. Effect of body mass index on operative outcome after robotic-assisted Ivor-Lewis esophagectomy: retrospective analysis of 129 cases at a single high-volume tertiary care center. Dis Esophagus 2017;30:1-7. [PubMed]
- Shridhar R, Hayman T, Hoffe SE, et al. Body mass index and survival in esophageal adenocarcinoma treated with chemoradiotherapy followed by esophagectomy. J Gastrointest Surg 2012;16:1296-302. [Crossref] [PubMed]
- Kayani B, Okabayashi K, Ashrafian H, et al. Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J Surg 2012;36:1785-95. [Crossref] [PubMed]


