A case-control study of the association between metabolic syndrome and colorectal cancer: a comparison of International Diabetes Federation, National Cholesterol Education Program Adults Treatment Panel III, and World Health Organization definitions
Introduction
An association between certain metabolic disorders, including high blood pressure, high serum triglyceride, high fasting blood glucose, low serum high-density lipoprotein, obesity, and cardiovascular diseases, have been known since the 1940s. Cardiovascular diseases indicate the malfunction of the cardiovascular system, mainly heart disease, the brain and kidney, blood vessels disease, and disease of peripheral arteries (1). Three decades ago [1980], few important risk factors were well established, and the condition called metabolic syndrome (MetS) (dysmetabolic syndrome or syndrome X) was introduced to indicate a group of metabolic risk factors that occur simultaneously in an individual (2). The clustering of these risk factors, like dyslipidemia, mainly high serum triglyceride and low serum high-density lipoprotein, impaired glucose tolerance, and hypertension, was first described by Reaven (2), and, subsequently, obesity was included (3). The World Health Organization (WHO) (4), the National Cholesterol Education Program Adults Treatment Panel III (NCEP ATP III) (5), and the International Diabetes Federation (IDF) criteria (6) proposed some individual criteria to describe this condition. In recent findings, the term ‘MetS’ is widely used in the literature of medical and science research, as well as in the lay press. The current interest was focused on the relationship between MetS and cancers.
Colorectal cancer (CRC) is one of the most commonly diagnosed solid tumours, and one of the main causes of cancer mortality around the world. CRC occurs when cancer develops in the surface of the large bowel, also called the large intestine (7). Worldwide, 9.4% of men and 10.1% of women, who had the incidence of cancer, were diagnosed with CRC. It is estimated that over 50% of people diagnosed with CRC will die of the disease, and it is the most common cancer in developed countries (8). In Peninsular Malaysia, CRC is the second most common cancer after breast (11.9% of all cases registered), the first among males (14.5%), and third among females (9.9%) (9). CRC incidence in Peninsular Malaysia is predominant among the Chinese, particularly among males (21.4% and 21.6%, respectively) (10).
Although there were a few studies previously conducted regarding the relationship between MetS and CRC, the evidence is still inconclusive since the relationship was population-specific. Since the Malaysian population differs from others in terms of dietary intake and nutritional status, this study aimed to measure the presence of MetS among the multi-ethnic in Malaysia using different definitions. It is hypothesised that this study may add new insight, from various definitions, to the existing evidences on the association between MetS and CRC.
Methods
This is a multi-centric hospital-based case control study. This study obtained ethical clearance from the Medical Ethics Committee of Faculty of Medicine and Health Sciences, University Putra Malaysia, the Clinical Research Centre of each hospital, and the Ministry of Health Research & Ethics Committee (MREC; NMRR-09-505-3994). The study was conducted in five local hospitals (Hospital Kuala Lumpur, Hospital Selayang, Hospital Putrajaya, Hospital Pulau Pinang, and Hospital Sultanah Aminah) that have a high number of diagnosed and treated CRC cases. Annually, approximately 80 new CRC cases are admitted in each hospital (11). This study was conducted from 1st December 2009 until 31st January 2012. Study respondents were recruited between 2010 and 2011.
The sample size was calculated using the Dupont formula for matched case-control studies. A total of 140 CRC cases were recruited in this study, after excluding those with missing data. Each case that was newly diagnosed with CRC, based on the colonoscopy and the histology report, was matched with two cancer-free controls (N=280) for age, gender, and ethnicity. Patients that had a history of cancer or were negative for malignancy were excluded from this study. Patients with CVD and renal failure were excluded as confounders. All patients who fulfilled criteria were recruited with written informed consent.
Interviewer administrative questionnaires were used to gather information on socio-demographic, physical activity, smoking status, alcohol consumption, and total energy intake, which were used as confounding variables for the association of components of MetS and MetS with CRC (12). All the study participants were ensured that they never received any form of dietary intervention prior to participating in this study, thus avoiding any information bias.
Twenty millilitre (mL) of venous blood sample was collected, processed, and analysed, as per standard procedure. Plasma fasting blood glucose, triglyceride, and HDL-cholesterol concentration were measured using an automated clinical chemistry analyser, Hitachi 902, based on hexokinase method, single point calibration, enzymatic colorimetric method, and rate method, respectively (13).
The cut off point for each of these analytes were based on the definitions proposed by the International Diabetes Federation (IDF) criteria (6), the National Cholesterol Education Program Adults Treatment Panel III (NCEP ATP III) (5), and modified World Health Organization (WHO) (4). Body composition measurements, such as height, weight, waist circumference, and blood pressure, were measured and categorised according to each criterion, as explained in the previous report (12).
SPSS version 2.1 was used to analyse the data. The data were described using descriptive statistics. Categorical variables were presented with frequencies and percentages; continuous variables were presented with means and standard deviations. Chi-square (χ2) was used to determine the association between socio-demographic and MetS, and its components based on each criterion. T-test was used to determine the difference of mean concentrations of components of MetS between cases and controls. Agreement between the three MetS definitions was analysed using the proportion of concordant cases and the Kappa index. Kappa index values above 0.81 were considered excellent, values from 0.61 to 0.80 were considered good, values from 0.41–0.60 were considered moderate, and values below 0.40 were considered weak (14). Conditional logistic regression was conducted using Cox-regression to measure the magnitudes of association of each component of MetS and MetS with CRC in a matched case-control study. Age, sex, ethnic, education background, energy intake, total physical activity level, current smoking status, and current alcohol consumption were entered in the model as confounding factors to measure the independent association between each component of MetS and MetS with CRC.
Results
Response rate
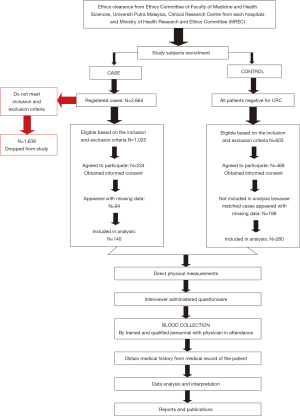
A total of 2,664 CRC cases were diagnosed in 2010 and 2011 in all five hospitals, based on the patients’ registration book in the colonoscopy room. About 1025 cases were eligible, based on the inclusion and exclusion criteria, and approached with a patients’ information sheet. However, only 234 cases agreed to participate in this study. For this study, only 140 cases were included in the analysis, because 94 cases appeared with missing data. The response rate of the study was 13.7%. On the other hand, all patients negative for CRC after a colonoscopy were screened for eligibility and matched for recruited cases. A total of 603 eligible controls were approached in this study, and 468 controls that matched the recruited cases were included in this study. The control samples were not random due to matching for age, gender, and ethnicity. The flow of recruitment of study respondents was illustrated in Figure 1.
Characteristics of study respondents
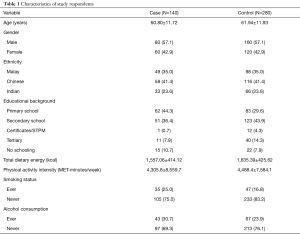
The mean age of cases (60.80±11.72 years) was slightly lower than those in the control group (61.94±11.93 years). Of 140 cases and 280 control subjects, 57.1% were males and the remaining 42.9% were females. The percentage of males in the case group out-numbered the females by 14.2%. In terms of ethnic variation, the incidence of CRC was highest among the Chinese (41.4%), followed by the Malays (35.0%) and the Indians (23.6%). The majority in the case group had primary education, while nearly 50% of the controls had secondary education. More case subjects (10.7%) had no schooling, compared to the control subjects (7.9%). Only one subject from the case group and 12 from the control group were educated up to certificate/STPM (0.7% and 4.3%, respectively). The rest of the participants, 7.9% of the cases and 14.3% of the controls, were educated up to the tertiary level. The total energy intake of the subjects in the case group was found to be lower than the control group (1,557.06±414.12 vs. 1,635.39±425.62 kcal). The total physical intensity in the case group was slightly lower compared to the controls (4,305.8±8,559.7 vs. 4,488.4±7,584.1 MET-minutes per day). The prevalence of smoking and alcohol consumption was higher among the cases compared to the controls (25.0% vs. 16.8% and 30.7% vs. 23.9%, respectively) (Table 1).
Full table
Prevalence of metabolic syndrome and individual components based on IDF definition
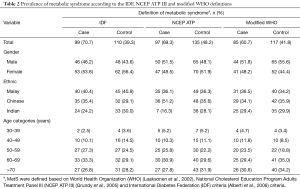
MetS, as defined by IDF, was diagnosed among 70.7% [99] of cases and among 39.3% [110] of controls. In both cases and controls, MetS was more prevalent among females (53.8% vs. 56.4%), when compared to males (46.2% vs. 43.6%). A higher proportion of the cases diagnosed with MetS were the Malays (40.4%), followed by the Chinese (35.4%) and the Indians (24.2%). Similarly, a higher proportion of the controls diagnosed with MetS were the Malays (40.9%), followed by the Indians (30.0%) and the Chinese (29.1%). A higher proportion of study subjects diagnosed with MetS based on the IDF definition were aged 60–69 years old (33.3% in the cases and 29.1% in the controls). The proportion of study subjects diagnosed with MetS increased with age advancement in both the cases and the controls (Table 2).
Full table
Prevalence of metabolic syndrome and individual components based on NCEP ATP III definition
Approximately 70% of the cases and 48.2% of the controls were diagnosed with MetS based on the NCEP ATP III definition. MetS was more prevalent among males (51.5%) than females in the cases; however, the proportion of the controls diagnosed with MetS was higher among females (51.9% and 48.1%, respectively). The Chinese (51.2%) had the highest prevalence among the cases, followed by the Malays (36.1%) and the Indians (16.3%). In the controls, the highest prevalence of MetS was observed among the Malays (36.3%), followed by the Chinese (35.6%) and the Indians (28.1%). MetS, as defined by the NECP ATP III, remained prevalent among cases aged 60–69 years (30.9%) while in the controls, MetS was prevalent among those aged 70 years and above (31.9%) (Table 2).
Prevalence of metabolic syndrome and individual components based on WHO definition
MetS was prevalent in males (51.8%) over females (48.2%) among the cases, while in the controls males were 55.6% and females were 44.4%, using the modified WHO definition. MetS was most prevalent among the Malays (36.5%), while the Chinese controls had the highest prevalence of MetS (35.9%). The Indians had the lowest prevalence of MetS in both the cases (29.4%) and the controls (29.9%). The cases aged ≥70 years and the controls aged 60–69 years had the highest prevalence of MetS (30.6% and 35.0%, respectively) (Table 2).
Prevalence of individual components of metabolic syndrome in the study groups based on IDF, NCEP ATP III and modified WHO definition
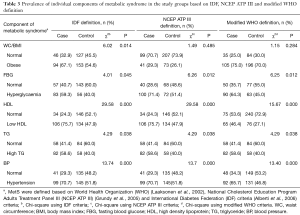
Abdominal obesity was significantly prevalent in 67.1% of cases based on the IDF criteria (χ2=6.02, P=0.014). The majority of the study subjects were not obese, according to the NCEP ATP III definition (70.7% in the cases and 73.9% in the controls). According to the modified WHO definition, the majority of the subjects were obese in both the cases (75.0%) and the control groups (70.0%) (Table 3). Significantly more of the cases than the controls had higher levels of FBG based on all three definitions of MetS. Nearly 60% of the cases had significantly high FBG according to the IDF definition (χ2=4.01, P=0.045), 71.4% according to the NCEP ATP III definition (χ2=6.26, P=0.012), and 64.3% according to the modified WHO definition (χ2=6.25, P=0.012) (Table 3). According to both the IDF and NCEP ATP III definitions, 75.7% of the cases had significantly low HDL cholesterol (χ2=29.58, P=0.000), whereas only 46.4% of the cases presented with low HDL cholesterol using the modified WHO definition (χ2=15.67, P=0.000). The majority of the study subjects appeared to have normal HDL levels, according to the modified WHO definition (53.6% in the cases and 72.9% in the controls) (Table 3). Almost 60% of the cases were significantly diagnosed with high serum TG level (χ2=4.29, P=0.038), based on all three definitions. High serum TG was less prevalent among the controls (40.0%) (Table 3). According to the IDF and NCEP ATP III definitions, the prevalence of hypertension was significantly higher among the cases (70.7%) and the controls (χ2=13.74, P=0.000). Hypertension was significantly more prevalent among the cases (65.7%), according to the modified WHO definition (χ2=13.40, P=0.000), compared to the controls (46.8%). The controls had moderately high prevalence of elevated BP, ranging from about 47–52%, as defined by all three definitions (Table 3).
Full table
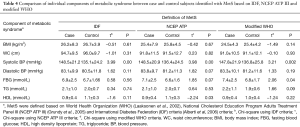
The cases generally diagnosed with MetS had a significantly higher mean of systolic BP (148.5±21.2 mmHg by the IDF and the NCEP ATPIII, and 147.8±21.9 mmHg by the modified WHO) and diastolic blood pressure (83.1±9.9 mmHg by the IDF, 83.8±9.7 mmHg by the NCEP ATP III and 83.3±10.1 mmHg) compared to the controls. The mean FBG (6.9±2.5, 7.1±2.5 and 7.4±2.5 mmol/L, respectively) and the mean TG (2.1±1.0 mmol/L by the IDF and the NCEP ATP III, and 2.2±1.1 mmol/L by the modified WHO) were found higher among the cases, but not significant. The mean HDL cholesterol was the lowest in the cases and similar in all definitions (0.9±0.4 mmol/L). The study findings showed that the mean values of BMI and waist circumference based on the IDF and the NCEP ATP III definitions were approximately the same between the cases and the controls with MetS (Table 4).
Full table
The study showed that MetS based on the IDF definition was successfully diagnosed in 70.7% of patients with NCEP ATP III MetS. On the other hand, 13.8% of the study subjects were categorized as normal by the NCEP ATP III, but were diagnosed with IDF MetS (sensitivity =83.7%, specificity =74.6%). Based on the kappa index, the IDF definition showed a good agreement with the NCEP ATP III definition, at 65.7±0.04 (P<0.001). The IDF MetS identified that 30.2% of study subjects had no modified WHO MetS and 22.5% of the study subjects who were identified as normal under the modified WHO definitions had IDF MetS (sensitivity =75.6%, specificity =71.9%). The kappa index showed a moderate agreement between the IDF definition and the modified WHO (47.4±0.04; P<0.001) (Table 5).

Full table
Association of metabolic syndrome and component of metabolic syndrome with CRC
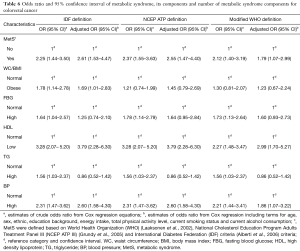
MetS based on the IDF definition significantly and independently increased odds of CRC more than two-fold (AOR =2.61; 95% CI, 1.53–4.47), while MetS based on the NCEP ATP III and WHO definitions significantly increased odds of CRC by 2.55 times and 1.79 times, independently (AOR =2.55; 95% CI, 1.47–4.40 and AOR =1.79; 95% CI, 1.07–2.99 respectively). Based on the IDF definition, the presence of abdominal obesity (AOR =1.69; 95% CI, 1.01–2.83), low HDL-cholesterol (AOR =3.79; 95% CI, 2.28–6.30), and hypertension (AOR =2.60; 95% CI, 1.58–4.30) were significantly associated with an increased odds of CRC. Based on the NCEP ATP III and WHO definitions, only having low HDL-cholesterol and being hypertensive increased the odds of CRC independently (Table 6).
Full table
Discussion
MetS has become an interest of studies in Malaysia, but to date no study has been published regarding the prevalence of MetS in CRC patients in Malaysia, although large studies have been conducted among healthy individuals (15,16) and on diabetes cases (17). The present study reported that the prevalence of MetS based on the IDF definition among CRC patients in Malaysia was higher than the MetS defined by the NCEP ATP III (70.7% vs. 69.3%) and followed by the modified WHO definition (60.7%). The authors hypothesized that the higher proportion of IDF MetS was due to the difference in the definition of abdominal obesity. The IDF proposed the presence of abdominal obesity as a prerequisite. Abdominal obesity for Malaysians was defined by the criterion for South Asians and Chinese (18). The criterion was consistent with the obesity criteria as proposed by the WHO Asia-Pacific Region (19). A similar trend was reported by (20), among type II diabetes patients, where 54.2% had MetS by the IDF definition and 50.5% had MetS by the NCEP-ATPIII definitions. In Europe, the MADRIC study (Madrid Riesgo Cardiovascular Study) involving 1,344 healthy subjects found that the prevalence of MetS was 24.6% using the NCEP ATP III definition and 30.9% using the IDF definition, after adjustments for age and gender. The MADRIC study found that the overall agreement between the NCEP-ATPIII and IDF definitions was at a satisfactory level, especially among women compared to men (κ: 0.92±0.07 vs. 0.66±0.06) (21). Consistently, this study showed a kappa index with a significant agreement between the IDF and NCEP ATP III definitions, but a moderate agreement between IDF and modified WHO definitions (κ: 0.66±0.04 vs. 0.47±0.04). There are four out of five identical components for MetS based on IDF and NCEP ATP III definitions. These gave a higher agreement between the IDF and NCEP ATP III definitions compared to the modified WHO definition.
However, these three definitions have their respective strengths and weakness. Since abdominal obesity is a prerequisite for the IDF definition, this is mainly preferential when diagnosing MetS among obese subjects. By measuring abdominal obesity in different ethnicities based on waist circumference measurements, the IDF definition becomes an appropriate measurement tool for epidemiologic data in Malaysia. The major disadvantage using the IDF definition is that a non-abdominally obese case could be excluded from MetS diagnosis, even with the presence of other criteria of the syndrome. The NCEP ATP III definition is the easiest method to diagnose MetS, and is used in many epidemiology studies as the preferred method. This is because of its flexibility in terms of the criteria used for diagnosis of MetS, where presence of any three out of five components of MetS fulfills the criteria. As for the modified WHO definition of MetS, type II diabetes is the precondition for the diagnosis. Thus, it could conclude that a non-diabetic case is healthy, although it is quite possible that the person has an underlying metabolic syndrome.
This study found that abdominal obesity significantly and independently increased, by almost 70%, CRC odds. From the concept of MetS, obesity is the major factor that induced insulin resistance, as insulin acts as a mitogen of tumour cell growth in vitro and as an important growth factor for colonic epithelial cells (22). In addition, abdominal obesity may alter the concentration of adipokines, including down-regulation of adiponectin and up-regulation of leptin, which were found to be significantly associated with colonic neoplasm. It is important to maintain the up-regulated adiponectin level, as it inhibits the signalling of the nuclear factor κB and promotes the cancer cell apoptosis. Besides that, adiponectin also inhibits the initiation of inflammation (23). The facts were supported by a case control study that found that having a higher waist circumference increased, by 82%, the CRC odds among postmenopausal women (24). In addition, the Cancer Prevention Study-II Nutrition Cohort in China reported that higher waist circumference both in men and women was significantly associated with increased CRC incidence (RR =1.68 in men and RR =1.75 in women) (25). A retrospective review of an institutional cancer database at the Michael E DeBakey Veterans Affairs Hospital [2002–2009] reported similar findings, where waist circumference was a better prognostic factor of CRC compared to body mass index (26).
In this study, the cases have a significantly higher prevalence of high FBG compared to the controls, but no significant association was found for CRC odds. There are only a few studies that have been conducted to determine the relationship between high blood glucose level and CRC odds in Malaysian populations (27); however, even fewer have determined the distribution of FBG across CRC site, stage, and other known risk factors in a multiple ethnic group (28). However, these studies only reported a moderate contribution to CRC odds. As reported in literature, T2DM was prevalent among Malaysians, exceeding 15% of the population (29), yet non-convincing evidences were found with the increased odds of CRC. These may be because the majority of CRC patients in this study had been diagnosed with T2DM more than a year, and they might control their sugar level by reduction of carbohydrate intake or exercising. This may be a reason the evidence between FBG and CRC risk is still controversial. In contrast to the authors’ findings, the Multi-Ethnic Cohort findings in Japanese Americans suggested that risk of CRC was increased by 15% in men with high serum glucose, whereas among women, the increased CRC risk was almost 50% with presence of high glucose level (30). The findings were consistent with earlier studies conducted in Koreans and Singaporean Chinese (31,32). He et al. also found high serum glucose to be significantly associated with the risk of colon cancer (RR =1.24) but not rectal cancer among African Americans (30). Contrary to these findings, an earlier report among African Americans found no association with CRC (33). A prospective study of CRC risk among women in the Women’s Health Initiative study, suggested that high serum glucose levels may be a stronger risk factor for CRC compared to insulin resistance, especially among postmenopausal women (34).
Low HDL cholesterol concentration significantly and independently increased odds of CRC almost four-fold. An experimental study suggested that low HDL-cholesterol promotes the tumour cells’ proliferation in vitro. HDL-cholesterol plays important roles in tumorigenesis through regulation of apoptosis or its influence on cell cycle entry, via a mitogen-activated protein kinase-dependent pathway (35). This study was supported by Jaggers et al., where low HDL cholesterol found an increased CRC risk of 25% (36). A large European study also found that the highest blood level of HDL cholesterol was significantly and independently protective against the risk for developing colon cancer, after adjusting for other confounding factors like poor diet and obesity. An increase of 16 mg/dL of plasma HDL concentration significantly and independently reduced the risk of colon cancer risk by 22%, after adjusting confounding factors such as weight, lifestyle, and dietary intake (37).
Consistent with this study’s findings, the CLUE II cohort of Washington County, Maryland found no association between TG and presence of CRC (38). In contrast to this finding, the Aerobics Centre Longitudinal Study in the United States reported that high TG increased the risk of CRC by 25% (36). A study that was carried out in China Medical University Hospital found that high TG levels independently and significantly increased the risk of CRC by almost 30% (39). Since obesity and T2DM are found to cause elevation of serum TG (40), high TG may contribute to CRC risk through simultaneous action of obesity and T2DM. This may be a reason this study found no independent contribution of CRC risk through high TG level.
Hypertension was found to increase CRC odds by almost three-folds in this study. Only a few studies have reported hypertension as a significant risk factor for CRC. MetS definition includes hypertension as one of the important components for its diagnosis; however, some studies hypothesized that the high correlation between MetS and hypertension was from conditions like hyperinsulinemia and insulin resistance (41). Despite the compelling evidence on the direct effect of hyperinsulinemia or insulin resistance, abdominal adiposity, which is etiologically different from the mechanism, may fluctuate the blood pressure and lead to hypertension (42). A study by the American Heart Association found a 35% increased risk of CRC associated with high blood pressure (43). This finding was confirmed by another prospective study (44); however, a study among smoking Finnish males did not support this association (45).
Measurement of MetS among this study’s subjects based on the IDF definition showed the strongest association with CRC. The odds estimates for any single component of MetS were significant for abdominal obesity, low-HDL cholesterol, and hypertension. This is biologically possible, because abdominal obesity was strongly correlated with every component of the MetS. Nevertheless, the ethnic-specific waist circumference cut-offs proposed by the IDF were appropriate and applicable to Malaysians (46,47). A meta-analysis revealed that MetS was strongly associated with increased risk of CRC both in men (33%) and women (41%). However, the main components of MetS that estimated the risk of CRC were obesity, high fasting blood glucose, and high blood pressure (48). IDF MetS was a significant risk factor of colon cancer, with almost twofold increased odds (35). In contrast, Liu et al. found no association between MetS and CRC, and only high blood glucose level was significantly associated with CRC (49). A case control study in Switzerland found MetS as risk factor for CRC in men, but not in women. The difference in the findings may be due to unmeasured other strong confounding factors of CRC risk, including smoking and poor diet, which differ by gender (50). It is hypothesised that the IDF definition that emphasised the importance of abdominal obesity with ethnic group stratification can be adopted worldwide, and proves convenient and useful in epidemiological studies and clinical practice. Despite the current significant findings, until randomised controlled trials confirm the finding, there is no established clinical or pharmacological treatment for the MetS among CRC cases especially for those with abdominal obesity low-HDL or hypertension. This retrospective observational study shows potentiality for unmeasured and residual confounding variables. However, in the analysis, the authors have controlled for all the possible confounders identified in previous studies.
Conclusions
In conclusion, the authors found that central obesity, hypertension, and low levels of HDL cholesterol were associated with increased odds of CRC, and only very high mean levels of metabolic factors confer increased odds of CRC. Since the IDF definition was more sensitive, this study concluded that the IDF definition is the most reliable reference to identify MetS in CRC among the Malaysian population. The components of MetS are modifiable risk factors; therefore, application of healthy behaviours, including exercise, weight control, and healthy dietary habits, are important in reducing these risk factors and thereby reducing the incidence of MetS and the risk of CRC. Since abdominal obesity was identified as significant in most of the studies on CRC, preventing obesity alone may be a potential strategy to control other risk factors, preventing the incidence of MetS and efficiently reducing the risk of CRC.
Acknowledgements
The authors thank the staff from the oncology, radiotherapy, and medical departments from the five public hospitals for their guidance and cooperation.
Funding: This work was funded by Fundamental Research Grant Scheme (FRGS; Vote No. 5523625; Project Code: 04-11-08-625FR).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Medical Ethics Committee of Faculty of Medicine and Health Sciences, University Putra Malaysia, the Clinical Research Centre of each hospital, and the Medical Research Ethics Committee of Ministry of Health, Malaysia (MREC; NMRR-09-505-3994). A written informed consent was obtained for all patients before performing study-related procedures.
References
- Kelly DL, McMahon RP, Liu F, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry 2010;71:304-11. [Crossref] [PubMed]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595-607. [Crossref] [PubMed]
- Isomaa B. A major health hazard: the metabolic syndrome. Life Sci 2003;73:2395-411. [Crossref] [PubMed]
- Laaksonen DE, Lakka HM, Salonen JT, et al. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002;25:1612-8. [Crossref] [PubMed]
- Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433-8. [Crossref] [PubMed]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world—wide definition. A consensus statement from the international diabetes federation. Diabet Med 2006;23:469-80. [Crossref] [PubMed]
- Raskov H, Pommergaard HC, Burcharth J, et al. Colorectal carcinogenesis--update and perspectives. World J Gastroenterol 2014;20:18151. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Lim GCC, Rampal S, Yahaya H. Cancer Incidence in Peninsular Malaysia, 2003-2005: The Third Report of the National Cancer Registry, Malaysia. National Cancer Registry; 2008.
- Omar ZA, Ali ZM, Tamin NSI. Malaysian Cancer Statistics-Data and Figure, Peninsular Malaysia 2006. National cancer registry, ministry of health Malaysia, 2006.
- Wendy L, Radzi M. New Registry: National Cancer Patient Registry-Colorectal Cancer. Med J Malaysia 2008;63:57-8. [PubMed]
- Ulaganathan V, Kandiah M, Zalilah M, et al. Colorectal cancer and its association with the metabolic syndrome: a Malaysian multi-centric case-control study. Asian Pac J Cancer Prev 2012;13:3873-7. [Crossref] [PubMed]
- Nguyen QM, Srinivasan SR, Xu JH, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: the Bogalusa Heart Study. Diabetes Care 2011;34:2603-7. [Crossref] [PubMed]
- Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994;308:1552. [Crossref] [PubMed]
- Mohamud WN. Prevalence of metabolic syndrome and its risk factors in adult Malaysians: results of a nationwide survey. Diabetes Res Clin Pract 2012;96:91-7. [Crossref] [PubMed]
- Rampal S, Mahadeva S, Guallar E, et al. Ethnic differences in the prevalence of metabolic syndrome: results from a multi-ethnic population-based survey in Malaysia. PLoS One 2012;7. [Crossref] [PubMed]
- Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 2010;29:289S-301S. [Crossref] [PubMed]
- Zimmet PZ, Alberti KG, Shaw JE. Mainstreaming the metabolic syndrome: a definitive definition. Med J Aust 2005;183:175-6. [PubMed]
- Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335-43. [Crossref] [PubMed]
- Luk AO, So WY, Ma RC, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 2008;31:2357-61. [Crossref] [PubMed]
- Martínez MA, Puig JG, Mora M, et al. Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) Study. Metabolism 2008;57:1232-40. [Crossref] [PubMed]
- Alemán JO, Eusebi LH, Ricciardiello L, et al. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology 2014;146:357-73. [Crossref] [PubMed]
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184-223. [Crossref] [PubMed]
- Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res 2008;68:329-37. [Crossref] [PubMed]
- Wang Y, Jacobs EJ, Patel AV, et al. A prospective study of waist circumference and body mass index in relation to colorectal cancer incidence. Cancer Causes Control 2008;19:783-92. [Crossref] [PubMed]
- Balentine CJ, Robinson CN, Marshall CR, et al. Waist circumference predicts increased complications in rectal cancer surgery. J Gastrointest Surg 2010;14:1669-79. [Crossref] [PubMed]
- Goh KL. Changing trends in gastrointestinal disease in the Asia–Pacific region. J Dig Dis 2007;8:179-85. [Crossref] [PubMed]
- Othman NH, Zin A. Association of colorectal carcinoma with metabolic diseases; experience with 138 cases from Kelantan, Malaysia. Asian Pac J Cancer Prev 2008;9:747-51. [PubMed]
- Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB, et al. Prevalence of diabetes in the Malaysian national health morbidity survey III 2006. Med J Malaysia 2010;65:180-6. [PubMed]
- He J, Stram D, Kolonel L, et al. The association of diabetes with colorectal cancer risk: the Multiethnic Cohort. Br J Cancer 2010;103:120-6. [Crossref] [PubMed]
- Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194-202. [Crossref] [PubMed]
- Seow A, Yuan JM, Koh WP, et al. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst 2006;98:135-8. [Crossref] [PubMed]
- Vinikoor LC, Long MD, Keku TO, et al. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev 2009;18:1239-42. [Crossref] [PubMed]
- Kabat GC, Kim MY, Strickler HD, et al. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br J Cancer 2012;106:227-32. [Crossref] [PubMed]
- Aleksandrova K, Jenab M, Bueno-de-Mesquita HB, et al. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 2014;29:261-75. [Crossref] [PubMed]
- Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer 2009;45:1831-8. [Crossref] [PubMed]
- Marotta T, Viola S, Ferrara F, et al. Improvement of cardiovascular risk profile in an elderly population of low social level: the ICON (Improving Cardiovascular risk profile in Older Neapolitans) study. J Hum Hypertens 2007;21:76-85. [Crossref] [PubMed]
- Tsilidis KK, Brancati FL, Pollak MN, et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control 2010;21:1-10. [Crossref] [PubMed]
- Lin WY, Liu CS, Li TC, et al. In addition to insulin resistance and obesity, hyperuricemia is strongly associated with metabolic syndrome using different definitions in Chinese populations: a population-based study (Taichung Community Health Study). Ann Rheum Dis 2008;67:432-3. [Crossref] [PubMed]
- Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care 2013;36:S233-9. [Crossref] [PubMed]
- Alfa-Wali M, Boniface S, Sharma A, et al. Metabolic syndrome (MetS) and risk of colorectal cancer (CRC): a systematic review and meta-analysis. World J Surg Med Radiat Oncol 2015;4:7.
- Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 2013;7:e330-41. [Crossref] [PubMed]
- Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 2003;107:1448-53. [Crossref] [PubMed]
- Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107:28-36. [Crossref] [PubMed]
- Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 2006;164:652-64. [Crossref] [PubMed]
- Tan CE, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182-6. [Crossref] [PubMed]
- Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care 2003;26:1380-4. [Crossref] [PubMed]
- Esposito K, Chiodini P, Capuano A, et al. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine 2013;44:634-47. [Crossref] [PubMed]
- Liu JJ, Druta M, Shibata D, et al. Metabolic syndrome and colorectal cancer: is hyperinsulinemia/insulin receptor-mediated angiogenesis a critical process? J Geriatr Oncol 2014;5:40-8. [Crossref] [PubMed]
- Chang LC, Wu MS, Tu CH, et al. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc 2014;79:961-9. [Crossref] [PubMed]