
What is the potential role of hepatic arterial infusion chemo-therapy in the current armamentorium against colorectal cancer
Introduction
Colorectal cancer (CRC) remains a major health problem in Europe and the United States. In Europe it is a common cancer (436,000 cases, 13.6% of the total) and the second most common cause of cancer-related mortality (212,000 cases, 12.3% of the total) (1). In the United States, CRC is the third most prevalent cancer and was estimated to have caused more than 50,000 deaths in 2010 (2).
The most common site of metastases from CRC is the liver. Approximately 20% of patients with CRC have clinically detectable liver metastases at initial presentation, and at least another 60% of patients will develop liver metastases during their disease course. Despite advances in surgical technique and expanded resectability criteria of liver metastases, radical surgical resection is not possible in 75% to 90% of patients with CRC (3).
Modern systemic chemotherapy regimens with or without biologic agents and liver-directed therapy may result in down staging liver metastases so that resection is possible. In this review, we will summarize the current role of hepatic arterial infusion (HAI) chemotherapy in increasing resection rate and decreasing recurrence after resection for patients with colorectal liver metastases.
Rationale of HAI chemotherapy
Colorectal liver metastases receive their blood supply almost exclusively from the hepatic artery, while blood flow to the normal liver parenchyma is mostly derived from the portal vein. Direct infusion of chemotherapeutic agents with high hepatic extraction via the hepatic artery can achieve prolonged drug exposure to tumor cells at a higher concentration. HAI also permits less exposure of normal liver to the drugs and reduces systemic toxicity.
HAI chemotherapy can be administered by a surgically implantable pump. Before pump placement, patients must have a carefully reviewed arteriogram or computed tomography angiogram to identify any aberrant hepatic anatomy. At the time of pump insertion, surgeons perform a cholecystectomy to prevent chemotherapy-induced cholecystitis. The pump's catheter is positioned at the junction of the proper and common hepatic arteries and threaded through the gastroduodenal, or celiac artery. The distal gastroduodenal artery, the right gastric artery, and small branches supplying the stomach and duodenum are ligated. The catheter is immobilized in the artery and the pump is placed in a subcutaneous pocket. During surgery, the pump is injected with a methylene blue dye to check for any extrahepatic perfusion. Postoperatively, a technetium 99m-labeled macroaggregated albumin scan is performed to confirm the pump's flow pattern and ensure no extrahepatic perfusion.
Several different chemotherapeutic agents have been administered via HAI in the treatment of colorectal liver metastases (4). Fluorodeoxyuridine (FUDR) is a useful agent for HAI because of its unique pharmacological properties. It has a short half-life (<10 minutes) and extensive first-pass extraction by the liver (94-99%) which results in an up to 100-400 fold estimated increase in hepatic exposure (5). In the United States FUDR is used most often for HAI, whereas 5-Fluorouracil (5-FU) is used in Europe and Japan (which only yields a 5-10 fold increase in hepatic exposure). Dexamethasone (20 mg) can be added with FUDR in order to reduce hepatotoxicity and increase efficacy (6,7). Irinotecan is not as well suited for regional HAI administration; it is converted to its active metabolite, SN-38, by hepatic metabolism. The non-linear pharmacokinetics of irinotecan predicts that at higher dose rates the clearance of the drug is diminished (8,9). Additionally, studies with HAI of irinotecan did not increase response or decrease toxicity (10,11). HAI of oxaliplatin has shown some increase in activity which will be covered in the next section. Using a human tumor colony forming assay, Kornmann et al. (12) detected significant concentration-dependent inhibition of colony formation after a 2 hours exposure to oxaliplatin, suggesting that patients with colorectal liver metastases may benefit from HAI with oxaliplatin. Dzodic et al. (13) investigated the pharmacokinetics of oxaliplatin after intravenous or HAI administration in a rabbit tumor model. They observed a significant pharmacokinetic advantage with HAI oxaliplatin with decreased peak platinum plasma concentrations, compared to the intravenous route. In addition, HAI of oxaliplatin showed a higher concentration in liver tumors (4.3 times that of the concentration found in normal liver tissue). HAI of oxaliplatin also exhibited a liver extraction ratio of 0.47 for oxaliplatin administered through the hepatic artery (14).
Does the addition of HAI to systemic chemo-therapy improve results in patients with colorectal liver metastases? Does it improve resectability?
Modern systemic chemotherapy has been used to produce tumor shrinkage allowing subsequent resection in about 15% to 30% of patients with initially unresectable colorectal liver metastases. Early randomized studies comparing HAI FUDR with systemic chemotherapy or best supportive care for CRC liver metastases demonstrated higher response rates for HAI chemotherapy, with response rates ranging 22% to 62% versus 9% to 25% in patients treated with systemic chemotherapy (15-24). The majority of the studies were small or allowed a crossover from systemic to HAI, so only three studies showed a significant overall survival benefit with HAI (20,21,24). These studies all used HAI alone without added systemic chemotherapy.
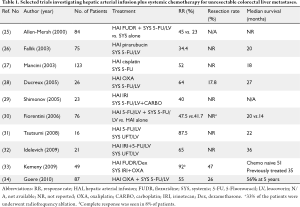
Available data suggests that HAI FUDR combined with systemic chemotherapy, including newer agents such as irinotecan and oxaliplatin, may be a promising approach to increase response and resectability rates in both untreated and previously treated patients with colorectal liver metastases. The combination of HAI and systemic treatment may also reduce the risk of extrahepatic progression. Table 1 shows selected studies investigating the role of HAI plus systemic chemotherapy as conversion therapy for patients with unresectable colorectal liver metastases (25-27,29-36). HAI FUDR/dexamethasone can be combined safely and effectively with systemic oxaliplatin and/or irinotecan-based regimens in this setting. At MSKCC, 49 patients who had initially unresectable liver metastases were treated with HAI FUDR/dexamethasone plus systemic oxaliplatin and irinotecan. Fifty-three percent of these patients were already treated with systemic therapy; therefore this therapy was second or third line. Ninety-two percent of patients had a response (8% complete, and 84% partial) and 47% of the patients were able to undergo resection (33). Many pre-operative studies do not describe why patients are unresectable. This study clearly showed the variables precluding resection: 24% of patients with all vessels involved, 73% with five or more liver lesions, 98% with bilobar disease, and 86% with six segments involved. Ninety percent of patients had a clinical risk score ≥3 (35). In patients who were chemotherapy naïve (n=23) 57% were able to undergo liver resection after treatment with HAI plus systemic therapy. All 23 patients had a response and the median survival was 50.8 months for these patients (33). For previously treated patients the response rate was 85% and the median survival was 35 months.
Full table
HAI of oxaliplatin plus systemic 5-FU/LV in patients with isolated unresectable colorectal liver metastases has been explored in several studies. Ducreux et al. (28) conducted a phase II study to evaluate concomitant administration of oxaliplatin via HAI and intravenous 5-FU/LV in 26 patients with inoperable isolated hepatic metastases from colorectal carcinoma. The objective response rate was 64% and five patients were able to undergo liver resection with curative intent. At a median follow-up of 23 months, the median overall and disease-free survival times were 27 and 27 months, respectively. Boige et al. (37) reviewed 87 patients who were treated with HAI oxaliplatin with intravenous 5-FU/LV for isolated unresectable colorectal liver metastases. Although about 79% of patients had previously received either systemic oxaliplatin or irinotecan, the treatment produced an objective response rate of 55%. After treatment, 26% of the patients were operated on with a curative intent. The resection rate was 53% in patients who received HAI as first-line and 19% in patients who received HAI after failure of prior systemic chemotherapy (P=0.008). Five-year overall survival was 56% in the surgery group versus 0% in the nonsurgery group (P<0.0001).
True Complete Responses
The use of preoperative HAI along with systemic chemotherapy may increase not only response rates, but also pathological complete response rates (38). In patients treated with systemic chemotherapy alone, Benoist et al. (39) examined 66 metastases that disappeared on helical computed tomography (CT) scans after chemotherapy. Persistent macroscopic disease was observed at surgery in 20 of 66 lesions. Resection of 15 lesions that disappeared showed viable tumor cells in 80%. Of the 31 sites not seen and left in place during surgery, 74% recurred. Therefore, only 17% were true complete responses. In a study from MSKCC, a total of 118 hepatic lesions that disappeared on CT scans after chemotherapy were evaluated. Sixty-eight of these lesions were resected, and 50 were followed clinically (40). Overall, 75 of 118 lesions (64%) were true complete responses, including 44 pathologic and 31 durable clinical complete responses. The true complete responses were more often seen in patients who had received prior HAI (87%) or who had no tumor seen on a magnetic resonance image (75%). The multivariate analysis revealed a significant association between HAI and the true complete responses. In the study by Elias et al. (38), patients who received HAI chemotherapy with oxaliplatin were more likely to have a pathological complete response compared with patients who received systemic chemotherapy alone (86% vs. 22%, P<0.02).
The use of HAI in the preoperative setting as first line therapy shows not only statistical improvement in survival but also seems to correlate with pathological response.
HAI plus systemic chemotherapy in second- line treatment
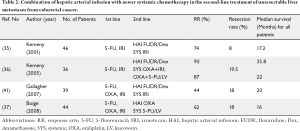
Response rates with systemic therapy alone are usually low when given as second line therapy. Consequently, survival after failing first line therapy is usually short. In a phase I study at Memorial-Sloan Kettering Cancer Center (MSKCC), the safety of the combination of HAI FUDR and dexamethasone plus systemic irinotecan in 46 patients with unresectable hepatic metastases from CRC was investigated (35). In this series, 40% of the patients had previously received irinotecan and none had received prior oxaliplatin. Although the main objective of the study was to evaluate the toxicity of the combined regimen, the treatment produced a high response rate (74%) and was well tolerated. Eight patients became amenable to hepatic cryosurgery. The median progression-free and overall survivals were 8.1 and 17.2 months for patients who did not undergo cryosurgery. In the group that underwent cryosurgery, median time to progression was 17.3 months. During a median follow-up of 26.4 months after surgery, only one patient died of disease. In another phase I experience using HAI FUDR and dexamethasone along with systemic oxaliplatin combinations (A: oxaliplatin and irinotecan or B: oxaliplatin and 5-FU/LV) in 36 patients with unresectable liver metastases, response and survival were again high (36). In this series, 89% of the patients had received prior chemotherapy, and 69.4% had prior irinotecan. The partial response rates were 90% and 87% for arms A and B, respectively. Seven patients in group A were able to undergo hepatic resection. The median survival time was 35.8 and 22 months for groups A and B, respectively. In a more recent study, the results in Arm A were confirmed. In 49 patients, response rate was 92% with a median survival of 41 months from the time of HAI therapy initiation, even though 53% were previously treated (36).
In a retrospective analysis, Gallagher et al. (41) reported a high partial response rate (44%) with systemic irinotecan plus HAI FUDR/dexamethasone in patients with metastatic CRC to the liver who progressed on oxaliplatin-based chemotherapy. The median survival was 20 months from the start of HAI therapy and 18% of patients were able to undergo surgical resection or ablation.
Administration of newer chemotherapy agents via HAI associated with systemic 5-FU-based therapy may be another approach in this setting. In a phase I study, 21 patients with hepatic metastases from CRC were treated with HAI oxaliplatin plus intravenous 5-FU/LV (42). The treatment regimen, which was administered every 3 weeks, consisted of 5-FU 600 mg/m2 and LV 200 mg/m2 intravenous combined with 25 mg/m2 oxaliplatin HAI with dose increments of 25 mg/m2. The limiting toxicities observed at an oxaliplatin dose of 150 mg/m2/cycle were leukopenia, occlusion of the hepatic artery, and acute pancreatitis. The recommended dose was 125 mg/m2 every 3 weeks. Overall, 24% of the patients achieved a complete response, with an overall response rate of 59%. The median time to progression had not been reached at the cutoff date, with a median follow-up of 6.7 months. In another phase I-II study conducted by Fiorentini et al. (43), 12 previously-treated (irinotecan, oxaliplatin and/or 5-FU/LV) patients with progressive CRC liver metastases received HAI with oxaliplatin as a 30 minute infusion every 3 weeks. Dose-limiting toxicity was observed at 175 mg/m2/cycle and consisted of occlusion of the hepatic artery, abdominal pain and severe hypotension. Following phase I, all patients were treated with 150 mg/m2 for six cycles. The overall response rate was 33% and the median survival was 13 months. In a small study reported by Mancuso et al. (44), patients treated with continuous HAI oxaliplatin (20 mg/m2/day × 5 days) alone showed a response rate of 46%, similar to response rates reported for HAI FUDR as monotherapy. Guthoff et al. (12) reported an overall response rate of 80% for patients treated with HAI using oxaliplatin in combination with 5-FU/LV and mitomycin C.
Boige et al. (37) investigated the activity of HAI oxaliplatin (100 mg/m2 over 2 hours) in combination with systemic 5-FU/LV in second-line chemotherapy for colorectal liver metastases previously treated with FOLFIRI (5-FU, LV and irinotecan), FOLFOX (5-FU, LV, and oxaliplatin), or both. They observed a response rate of 62%, which led to R0 resection or radiofrequency ablation in 18% of patients.
A newer prospective study at MSKCC randomized patients to receive Bevacizumab in combination with HAI FUDR and systemic therapy. Of the chemo naïve patients on the non Bev arm, 67% were converted to resectable status.
These studies strongly suggest that HAI therapy should be considered as chemotherapy in the second-line treatment of patients with colorectal liver metastases (Table 2). With the addition of HAI, patients are more likely to undergo liver resection even after having failed first-line therapy.
Full table
Is there a role for HAI in adjuvant treatment after hepatic resection?
Although resection of colorectal liver metastases remains the only curative option, nearly 70% of patients develop recurrence after surgery, which occurs most commonly within two years. Thus, there is a rationale for adjuvant chemotherapy after liver resection. Adjuvant systemic chemotherapy with 5-FU/LV showed an increase in disease-free but not overall survival (45,46). FOLFIRI did not significantly improve outcomes compared with 5-FU/LV (47). There is no randomized data supporting the use of adjuvant FOLFOX or another oxaliplatin-based chemotherapy after liver resection. In the light of these data the determination of an optimal adjuvant systemic chemotherapy regimen is unclear (48).
Since the majority of recurrences occur in the liver, HAI therapy after liver resection is an option for patients with CRC. Implantation of the HAI pump can be done in conjunction with liver resection. Early randomized studies comparing HAI plus or minus systemic 5-FU-based chemotherapy after liver resection showed that combined therapy significantly improved disease-free survival (49-52). Other studies suggest that modern systemic chemotherapy (i.e., irinotecan and oxaliplatin) and HAI can be safely integrated in order to achieve better overall outcomes (Table 3). In a phase I/II study, HAI FUDR/dexamethasone in combination with intravenous irinotecan after resection of hepatic metastases from CRC was investigated. Treatment was well tolerated and adverse events were manageable. At a median follow-up of 26 months, the 2-year survival was 82% and 2-year PFS was 47%. Additionally promising survival data was reported in a recent phase I study combining HAI FUDR/dexamethasone with systemic oxaliplatin-based chemotherapy in 35 patients with resected liver metastases. Overall survival was 84% at 4 years and progression-free survival was 81% at 1 year, 58% at 2 years, and 50% at 3, 4 and 5 years (54).

Full table
In a newer study, 73 patients were treated with HAI FUDR/dexamethasone plus intravenous oxaliplatin- or irinotecan-based regimens with or without bevacizumab after resection of liver metastases (56). Although 48% of the patients had poor prognostic indicators, including 81% of patients with more than one hepatic metastasis, very satisfactory survival results were reported (4-year survival of 85% in no bevacizumab arm and 81% in bevacizumab arm).
In a more recent intergroup trial, HAI FUDR alternating with systemic oxaliplatin and capecitabine was assessed after resection of colorectal liver metastases (55). After a median follow-up of 4.8 years, 55% of the patients recurred. Median time to recurrence was 2.7 years. At 2 years after surgery, 88% of the patients were alive. These promising results prompted the authors to open a larger phase III study comparing capecitabine and oxaliplatin with or without HAI FUDR, but the study was closed early due to poor accrual (57).
House et al. retrospectively analyzed 250 patients who underwent resection of colorectal liver metastases between 2001 and 2005 and received either adjuvant HAI FUDR with systemic chemotherapy (FOLFOX or FOLFIRI), or adjuvant systemic chemotherapy alone. The 5-year liver-recurrence free survival (RFS), overall RFS, and overall survival in the HAI group were 77%, 48%, and 75%, respectively versus 55%, 25%, and 55% in the chemotherapy alone group (P<0.01). The multivariate analysis also revealed adjuvant treatment with HAI and systemic therapy as an independent factor for longer disease free survival (P<0.01) (Accepted for publication in Annals of Surgery, 2011).
Complications of HAI
The complications of HAI may be technical, drug-related or a combination of both. In 2001, Barnett et al. (58) reviewed 4580 cases that were treated with HAI for colorectal liver metastases. 5-FU and FUDR were the most commonly used drugs for HAI. The most common toxicities were gastrointestinal symptoms (25%), chemical hepatitis (22%), and bone marrow inhibition (9%). The most common catheter-related complications were catheter displacement (7%), hepatic artery occlusion (6%), and catheter thrombosis (5%). The technical complication rates have been shown to drop with increased surgical experience and improvements in pump design. Allen et al. (59) reported on pump complications in 544 patients treated at MSKCC between 1986 and 2001. The overall pump complication rate was 22%. These complications consisted of arterial thrombosis (6%), extrahepatic perfusion (3%), incomplete hepatic perfusion (2%), and hemorrhage (2%). However, the complications during the earlier half of the study period (1986-1993) were significantly higher (25%) than the later half of the study time (1994-2001, 18%, P=0.05). The majority of complications were also salvaged, with 80% of pumps functioning for a minimum of 2 years. Overall pump failure rates were 9% at 1 year and 16% at two years.
Hepatobiliary toxicity is the most serious and dose-limiting complication of HAI. It occurs at a higher incidence with FUDR (60). Elevation of serum transaminase levels is often the first sign of hepatotoxicity. Increases in alkaline phosphatase and bilirubin are more serious and show evidence of more significant hepatic damage. Therefore, changes in liver functions should be monitored regularly during treatment with HAI FUDR. A dose-adjusting algorithm has been devised based on changes in liver function tests (61). Addition of dexamethasone to HAI of FUDR may reduce incidence of bilirubin elevation and also increase the rate of treatment response as demonstrated by Kemeny et al. (6,7). In the adjuvant pump studies at MSKCC, more than twofold increase in alkaline phosphatase levels was observed in 14% to 43% of patients. Total bilirubin elevation > 3.0 mg/dL was seen in 0 to 19%, and biliary stents were placed in 0 to 8%. A higher incidence of biliary toxicity was seen in the study where FUDR dose was 0.14 (as compared to 0.12 in the newer studies). In a new study that was recently published in JCO, patients were randomized to receive Bev versus no Bev in addition to HAI + FOLFOX or FOLFIRI. In the group that received Bev, bilirubin ≥3 mg/dL was seen in 5 of 35 versus zero of 38 patients (P=0.02) and biliary stents were placed in four versus zero patients (P=0.05). This study was terminated early due to biliary toxicity.
Biliary sclerosis is not observed with HAI of 5-FU (58) which tends to associate more with increased risk of myelosupression (58). Therefore, one logical approach to reduce biliary toxicity is to alternate between HAI FUDR and HAI 5-FU. Davidson et al. (62) used HAI FUDR at a dose of 0.1 mg/kg/day for seven days followed by HAI boluses of 5-FU 15 mg/kg on days 14, 21, and 28. With this schedule, 12 (21%) patients had temporary liver enzyme elevations and only 2 patients (3.5%) developed biliary sclerosis. In another study, HAI FUDR was administered for seven days, and HAI 5-FU bolus was given on days 15, 22 and 29, with the cycle repeated every 35 days. None of the patients in this study had treatment terminated because of hepatobiliary toxicity (63).
Conclusions
Available data and literature demonstrates that HAI may have a definitive role in successful treatment of patients with hepatic metastases from CRC. HAI can be combined safely and effectively with modern systemic chemotherapy in neoadjuvant (conversion therapy), second-line and adjuvant treatment of selected patients. On the other hand, concerns about technical problems and potential toxicity of the treatment may discourage oncologists from using HAI. However, improvement in surgical techniques and the development of modern implantable pumps have decreased technical complications and improved patient tolerability of treatment. Alternative treatment modalities are needed to increase survival rates for patients with colorectal liver metastases. The use of HAI in conjunction with systemic chemotherapy seems to be a promising approach for these patients. Further large prospective randomized studies could clarify the exact role of HAI for neoadjuvant, second-line or adjuvant treatment of colorectal liver metastases.
Footnote
No potential conflict of interest.
References
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765-781. [PubMed]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [PubMed]
- Kingham TP, D'Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol 2010;102:988-995. [PubMed]
- Kelly RJ, Kemeny NE, Leonard GD. Current strategies using hepatic arterial infusion chemotherapy for the treatment of colorectal cancer. Clin Colorectal Cancer 2005;5:166-174. [PubMed]
- Kemeny N, Seiter K, Niedzwiecki D, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer 1992;69:327-334. [PubMed]
- Kemeny N, Conti JA, Cohen A, et al. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol 1994;12:2288-2295. [PubMed]
- Iyer L, Ratain MJ. Clinical pharmacology of camptothecins. Cancer Chemother Pharmacol 1998;42:S31-S43. [PubMed]
- Abigerges D, Chabot GG, Armand JP, Hérait P, Gouyette A, Gandia D. Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 1995;13:210-221. [PubMed]
- van Riel JM, van Groeningen CJ, de Greve J, Gruia G, Pinedo HM, Giaccone G. Continuous infusion of hepatic arterial irinotecan in pretreated patients with colorectal cancer metastatic to the liver. Ann Oncol 2004;15:59-63. [PubMed]
- van Riel JM, van Groeningen CJ, Kedde MA, et al. Continuous administration of irinotecan by hepatic arterial infusion: a phase I and pharmacokinetic study. Clin Cancer Res 2002;8:405-412. [PubMed]
- Kornmann M, Fakler H, Butzer U, Beger HG, Link KH. Oxaliplatin exerts potent in vitro cytotoxicity in colorectal and pancreatic cancer cell lines and liver metastases. Anticancer Res 2000;20:3259-3264. [PubMed]
- Dzodic R, Gomez-Abuin G, Rougier P, et al. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs 2004;15:647-650. [PubMed]
- Guthoff I, Lotspeich E, Fester C, et al. Hepatic artery infusion using oxaliplatin in combination with 5-fluorouracil, folinic acid and mitomycin C: oxaliplatin pharmacokinetics and feasibility. Anticancer Res 2003;23:5203-5208. [PubMed]
- Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 1987;107:459-465. [PubMed]
- Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg 1987;206:685-693. [PubMed]
- Hohn DC, Stagg RJ, Friedman MA, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol 1989;7:1646-1654. [PubMed]
- Wagman LD, Kemeny MM, Leong L, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol 1990;8:1885-1893. [PubMed]
- Martin JK Jr, O'Connell MJ, Wieand HS, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer. A randomized trial. Arch Surg 1990;125:1022-1027. [PubMed]
- Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol 1992;10:1112-1118. [PubMed]
- Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 1994;344:1255-1260. [PubMed]
- Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol 2000;18:243-254. [PubMed]
- Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003;361:368-373. [PubMed]
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-1403. [PubMed]
- Allen-Mersh TG, Glover C, Fordy C, Mathur P, Quinn H. Randomized trial of regional plus systemic fluorinated pyrimidine compared with systemic fluorinated pyrimidine in treatment of colorectal liver metastases. Eur J Surg Oncol 2000;26:468-473. [PubMed]
- Fallik D, Ychou M, Jacob J, et al. Hepatic arterial infusion using pirarubicin combined with systemic chemotherapy: a phase II study in patients with nonresectable liver metastases from colorectal cancer. Ann Oncol 2003;14:856-863. [PubMed]
- Mancini R, Tedesco M, Garufi C, et al. Hepatic arterial infusion (HAI) of cisplatin and systemic fluorouracil in the treatment of unresectable colorectal liver metastases. Anticancer Res 2003;23:1837-1841. [PubMed]
- Ducreux M, Ychou M, Laplanche A, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol 2005;23:4881-4887. [PubMed]
- Shimonov M, Hayat H, Chaitchik S, Brener J, Schachter P, Czerniak A. Combined systemic chronotherapy and hepatic artery infusion for the treatment of metastatic colorectal cancer confined to the liver. Chemotherapy 2005;51:111-115. [PubMed]
- Fiorentini G, Cantore M, Rossi S, et al. Hepatic arterial chemotherapy in combination with systemic chemotherapy compared with hepatic arterial chemotherapy alone for liver metastases from colorectal cancer: results of a multi-centric randomized study. In Vivo 2006;707-9.
- Tsutsumi S, Yamaguchi S, Tsuboi K, et al. Hepatic arterial infusion combined with oral UFT/UZEL systemic chemotherapy for unresectable liver metastasis of colorectal cancer. Hepatogastroenterology 2008;55:1419-1422. [PubMed]
- Idelevich E, Greif F, Mavor E, et al. Phase II study of UFT with leucovorin plus hepatic arterial infusion with irinotecan, 5-fluorouracil and leucovorin for non-resectable liver metastases of colorectal cancer. Chemotherapy 2009;55:76-82. [PubMed]
- Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-3471. [PubMed]
- Goéré D, Deshaies I, de Baere T, et al. Prolonged survival of initially unresectable hepatic colorectal cancer patients treated with hepatic arterial infusion of oxaliplatin followed by radical surgery of metastases. Ann Surg 2010;251:686-691. [PubMed]
- Kemeny N, Gonen M, Sullivan D, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol 2001;19:2687-2695. [PubMed]
- Kemeny N, Gonen M, Sullivan D, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol 2005;23:4888-4896. [PubMed]
- Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219-226. [PubMed]
- Elias D, Goere D, Boige V, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol 2007;14:3188-3194. [PubMed]
- Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006;24:3939-3945. [PubMed]
- Auer RC, White RR, Kemeny NE, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer 2010;116:1502-1509. [PubMed]
- Gallagher DJ, Capanu M, Raggio G, Kemeny N. Hepatic arterial infusion plus systemic irinotecan in patients with unresectable hepatic metastases from colorectal cancer previously treated with systemic oxaliplatin: a retrospective analysis. Ann Oncol 2007;18:1995-1999. [PubMed]
- Kern W, Beckert B, Lang N, et al. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann Oncol 2001;12:599-603. [PubMed]
- Fiorentini G, Rossi S, Dentico P, et al. Oxaliplatin hepatic arterial infusion chemotherapy for hepatic metastases from colorectal cancer: a phase I-II clinical study. Anticancer Res 2004;24:2093-2096. [PubMed]
- Mancuso A, Giuliani R, Accettura C, et al. Hepatic arterial continuous infusion (HACI) of oxaliplatin in patients with unresectable liver metastases from colorectal cancer. Anticancer Res 2003;23:1917-1922. [PubMed]
- Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24:4976-4982. [PubMed]
- Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906-4911. [PubMed]
- Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol 2009;20:1964-1970. [PubMed]
- Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol 2010;28:2300-2309. [PubMed]
- Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy- an intergroup study. J Clin Oncol 2002;20:1499-1505. [PubMed]
- Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-2048. [PubMed]
- Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005;352:734-735. [PubMed]
- Lygidakis NJ, Sgourakis G, Vlachos L, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology 2001;48:1685-1691. [PubMed]
- Kemeny N, Jarnagin W, Gonen M, et al. Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol 2003;21:3303-3309. [PubMed]
- Kemeny N, Capanu M, D'Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol 2009;20:1236-1241. [PubMed]
- Alberts SR, Roh MS, Mahoney MR, et al. Alternating systemic and hepatic artery infusion therapy for resected liver metastases from colorectal cancer: a North Central Cancer Treatment Group (NCCTG)/ National Surgical Adjuvant Breast and Bowel Project (NSABP) phase II intergroup trial, N9945/CI-66. J Clin Oncol 2010;28:853-858. [PubMed]
- Kemeny NE, Jarnagin WR, Capanu M, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol 2011;29:884-889. [PubMed]
- Hubbard JM, Alberts SR. Treatment of liver-limited metastatic colorectal cancer. Cancer J 2010;16:235-240. [PubMed]
- Barnett KT, Malafa MP. Complications of hepatic artery infusion: a review of 4580 reported cases. Int J Gastrointest Cancer 2001;30:147-160. [PubMed]
- Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg 2005;201:57-65. [PubMed]
- Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther 2009;8:1015-1025. [PubMed]
- Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 2003;8:553-566. [PubMed]
- Davidson BS, Izzo F, Chase JL, et al. Alternating floxuridine and 5-fluorouracil hepatic arterial chemotherapy for colorectal liver metastases minimizes biliary toxicity. Am J Surg 1996;172:244-247. [PubMed]
- Stagg RJ, Venook AP, Chase JL, et al. Alternating hepatic intra-arterial floxuridine and fluorouracil: a less toxic regimen for treatment of liver metastases from colorectal cancer. J Natl Cancer Inst 1991;83:423-428. [PubMed]


