Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum
Introduction
Gastrointestinal stromal tumors (GISTs) are rare, yet they are the most common mesenchymal neoplasm in the gastrointestinal tract with an annual incidence in the United States of 3,300–6,000 new cases a year (1). Postulated to arise from the interstitial cell of Cajal, 75–80% of cases have mutations in the C-KIT proto-oncogene in exons 9, 11, 13, or 17, while an additional 10% possess platelet-derived growth factor receptor (PDGFR) mutations (2-4). A clear understanding of the tumor biology has led to improved treatment of these sarcomatous neoplasms of the gastrointestinal tract. In particular, knowledge of these genetic abnormalities led to the use of multi-targeted tyrosine kinase inhibitors (imatinib, sunitinib, regorafenib), which have revolutionized the treatment of solid tumors (5-8).
Up to 90% of these tumors arise from the stomach and small intestine while the remaining arise from the esophagus, colon, and rectum (9-12). Nearly all treatment decisions in patients with GISTs are predicated on the study of gastric and small bowel GISTs. Based on several randomized controlled trials of GISTs, the majority of which are of gastric and small bowel origin, the most important factors that have been shown to impact clinical outcome are organ of origin, tumor size in largest dimensions, and mitotic rate (10,11,13,14).
There is a paucity of medical literature describing GISTs that arise from uncommon locations of the gastrointestinal tract. Thus, there is a need for a better characterization of this histologic type of tumor by primary organ site to improve patient care. The purpose of this study was to provide a comprehensive, detailed description of colon and rectal GISTs and to compare their behavior to that of the more commonly described gastric GIST.
Methods
Patient population
This study was reviewed by the Yale Institutional Review Board and deemed exempt from review as a secondary data analysis. The American College of Surgeons (ACS) National Cancer Data Base (NCDB) was reviewed to identify patients from 2006–2013 with gastric, colon (including rectosigmoid junction), or rectal primary tumors with histologically defined GIST by ICD-O-3 code 8936.
Study variables
From the NCDB, variables were gathered on patient demographics, cancer identification, and treatment. Patient demographic variables included sex, age, race, Hispanic ethnicity, insurance, median income, Charlson-Deyo comorbidity score, facility type, and facility location. Tumor characteristics included primary site, tumor size, and TNM clinical M stage. Treatment variables included systemic therapy administered (coded as single-agent and multiple-agent chemotherapy, with single-agent representing standard of care targeted biologic therapy, namely imatinib), primary surgery, surgical margins, and unplanned readmission with 30 days of surgery. Primary surgery was categorized as local (including local excision and local ablation) vs. radical (including any partial or total gastrectomy, partial or total colectomy, proctocolectomy, proctectomy, and wedge or segmental resection, as this coding includes anterior resection, Hartmann operation, low anterior resection, transsacral rectosigmoidectomy, and total mesorectal excision).
Statistical analysis
Patient, cancer, and treatment characteristics are reported as frequencies for defined categorical variables and as means (SD) for continuous variables. Categorical variables were compared using chi-square or Fisher’s exact test, and continuous variables were compared using ANOVA. For purposes of survival data, gastric and colorectal GISTs were 1:1 propensity matched on age, gender, race, insurance, median income, facility location, facility type, and Charlson-Deyo score. The propensity match was conducted using a previously described SAS macro (15). After matching, there were not statistically significant differences between gastric and colorectal GISTs in these characteristics. In order to account for immortal time bias, patients who died within 90 days of primary surgery were excluded from survival analysis. Primary outcome was overall survival, reported using Kaplan-Meier survival curve and log-rank test. Survival analysis was additionally conducted by multivariate Cox regression. Overall survival by primary site was adjusted for tumor size, clinical M stage, systemic therapy, surgery, surgical margins, and year of diagnosis. Overall survival by treatment type was adjusted for tumor size, clinical M stage, surgical margin, and year of diagnosis. A P value less than 0.05 was considered statistically significant, and all statistical tests were two-sided. All statistical analyses were performed using SAS software, version 9.4 (SAS, Institute Inc., Cary, NC, USA).
Results
Descriptive
A total of 12,093 GIST patients were identified. Among them, 11,302 (93.5%) were gastric GISTs, 398 (3.3%) colon GISTs (including 371 colon and 27 rectosigmoid), and 393 (3.2%) rectal GISTs.
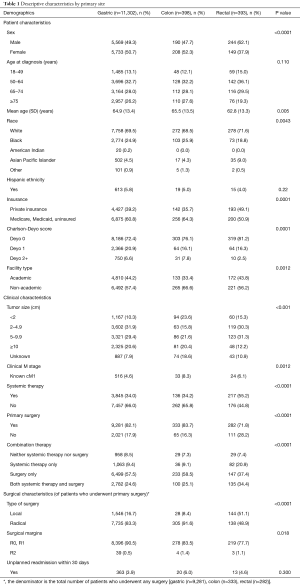
Descriptive characteristics are shown in Table 1. Rectal GISTs predominantly occurred in male patients (62.1%), while gastric and colon GISTs were more evenly split (49.3% male for gastric, 47.7% male for colon). Rectal GISTs were diagnosed in younger patients than gastric and colon GISTs (mean age 62.8 vs. 64.9 vs. 65.5 years, P=0.005). Rectal GISTs were less likely to present at ≥10 cm in size than gastric and colon GISTs (12.2% vs. 20.6% vs. 20.4%, P<0.0001). Gastric GISTs were less likely to present with clinical metastatic disease than colon or rectal GISTs (4.6% vs. 8.3% vs. 6.1%, P=0.0012).
Full table
With regards to therapy, rectal GISTs compared to gastric and colon GISTs were more likely to receive systemic therapy (55.2% vs. 34.0% vs. 34.2%, P<0.0001) and to receive a combination of systemic therapy and surgery (34.4% vs. 24.6% vs. 25.1%, P<0.0001). Rectal GISTs were also more likely to undergo a local rather than radical surgery on their primary tumor than gastric and colon GISTs (51.1% vs. 16.7% vs. 8.4%, P<0.0001). Gastric GISTs were more likely to achieve an R0 or R1 resection than either colon or rectal GISTs (90.5% vs. 83.5% vs. 77.7%, P=0.018). There was no statistical difference in the rate of 30-day unplanned readmission between primary sites (P=0.300).
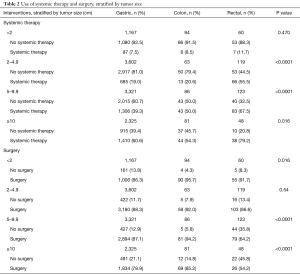
Table 2 shows the utilization of systemic therapy and surgery stratified by tumor size. Rectal GISTs were consistently more likely to receive systemic therapy at all tumor sizes, reaching statistical significance in tumor sizes greater than 2 cm (2–4.9 cm, P<0.0001; 5–9.9 cm, P<0.0001; ≥10 cm, P=0.016). Rectal GISTs were also less likely to receive primary surgery than gastric or colon GISTs except at the smallest tumor sizes.
Full table
Kaplan-Meier survival
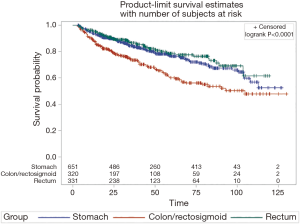
After landmarking at 90 days for immortal time bias, survival data was available on 691 colon and rectal GIST patients (87.4%). Gastric GISTs were propensity matched to colon and rectal GISTs 1:1 on age, sex, race, insurance, median income, facility location, facility type, and Charlson-Deyo score. Figure 1 demonstrates Kaplan-Meier curves of overall survival by primary site. Median survival for gastric GISTs was significantly prolonged compared to colon GISTs (88.51 vs. 71.3 months, log-rank P=0.0003), and similarly median survival for rectal GISTs was significantly improved compared to colon GISTs (85.7 vs. 71.3 months, log-rank P<0.0001), yet survival did not significantly differ between gastric and rectal GISTs (log-rank P=0.9995).
Cox regression
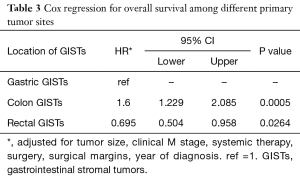
Cox regression adjusted for covariates by primary site shown in Table 3 demonstrated worse overall survival for colon GISTs compared to gastric GISTs (HR =1.6, P=0.0005) and improved overall survival for rectal GISTs compared to gastric GISTs (HR =0.695, P=0.0264).
Full table
Cox regression adjusted for covariates by the addition of adjuvant systemic therapy to surgery in Table 4 demonstrated a significant improvement in overall survival only in rectal GISTs (HR =0.47, P=0.042). Gastric GISTs also demonstrated an improvement in overall survival with adjuvant systemic therapy at all tumor sizes and stratified by tumor size, reaching statistical significance in tumor sizes ≥10 cm (HR =0.34, P=0.046). Colon GISTs did not show an improvement in overall survival and demonstrated worse overall survival with the addition of adjuvant therapy in tumors <5 cm (HR =3.41, P=0.032). Pre-operative and post-operative systemic therapy were also individually analyzed and did not demonstrate a significant improvement in overall survival for gastric, colon, or rectal GISTs (data not shown).
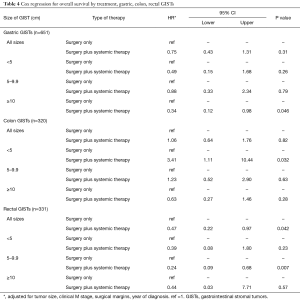
Full table
Discussion
GISTs which arise in the colon and rectum are rare, collectively accounting for only 7% and 5% of all histologic cases, respectively (12). In this retrospective study utilizing the NCDB, we compare 11,302 gastric GISTs to 791 colon and rectal GISTs to perform the largest study to date that characterizes these rare anatomic variants of GISTs. We demonstrate unique tumor biology and patterns of care for gastric, rectal, and colon GISTs, with improved survival and response to adjuvant systemic therapy for tumors of rectal origin.
There are multiple clinical characteristics that distinguish colon and rectal GISTs from gastric GISTs. With regards to survival, GISTs arising in the colon and rectum show different patterns compared to the paradigms of other cancers. In contradistinction to colorectal adenocarcinoma, rectal GISTs fare better than colon GISTs (16). Our unadjusted survival analyses show that patients with rectal GISTs demonstrated a prolonged overall survival compared to colon GISTs, with a mean survival time of 85.7 vs. 71.3 months, log-rank P<0.0001. And while GISTs arising from the stomach are traditionally considered a favorable prognostic feature, our data shows that tumors of rectal origin fare better than those of gastric origin (17). In multivariate Cox regressions adjusted for clinical M stage, tumor size, surgical margins, and treatment strategy, rectal GISTs further demonstrated improved overall survival compared to gastric GISTs (HR =0.695, P=0.0264). On the contrary, patients with colon GISTs demonstrate worse overall survival compared to gastric GISTs while still receiving comparable frequencies of systemic therapy and adjusted for tumor size, clinical M stage, surgical margins, and treatment strategy (HR =1.6, P=0.0005). This is consistent with previous findings from the Surveillance Epidemiology and End Result (SEER) database, which, in a series of 126 colon and 135 rectal GISTs, demonstrated that colon GISTs have worse disease specific survival compared to rectal GISTs (18). Thus, current standard systemic therapies (i.e., imatinib, sunitinib, regorafenib) for GIST appear less efficacious for tumors arising from the colon as opposed to the stomach suggesting a difference in tumor biology based on organ of origin.
Currently there are no guidelines individualized to GISTs by primary site, and our data shows that there are clear differences in provider use of systemic therapy and surgery based on organ. A higher percentage of rectal GISTs were treated with systemic therapy overall compared to gastric and colon GISTs (55.2% vs. 34.0% vs. 34.2% respectively, P<0.0001). In addition, we also show that a higher percentage of rectal GISTs were treated with a combination of systemic therapy and surgery (34.4% vs. 24.6% vs. 25.1% respectively, P<0.0001), which was a trend that was consistent at all tumor sizes. Colon GISTs, on the other hand, received systemic therapy at a high, yet comparable, frequency to the more common gastric GISTs, when analyzing both systemic treatment overall and multi-modality approaches. With regards to the use of adjuvant systemic therapy, rectal GISTs demonstrated an improvement in adjusted overall survival at all sizes, with over a 50% reduction in risk of death (HR =0.47, P=0.042), a trend that was also seen when stratified by tumor size. Gastric GISTs, while similarly demonstrating an improvement in overall survival with adjuvant systemic therapy, only reached statistical significance in tumors ≥10 cm (HR =0.34, P=0.046). This is not inconsistent with the current standard of care for GISTs, where the decision to use adjuvant systemic therapy is based on prognostic factors, including maximum tumor dimensions (5). Colon GISTs, however, did not show an improvement in overall survival with adjuvant systemic therapy (HR =1.06, P=0.82), and actually demonstrated significantly worse overall survival with adjuvant systemic therapy in tumors <5 cm (HR =3.41, P=0.032). At the largest tumor sizes ≥10 cm, colon GISTs were associated with an improvement in overall survival, but failed to achieve statistical significance (HR =0.63, P=0.28). Taken together, this suggests that patients with colonic GISTs are over treated with tyrosine kinase inhibitors. Standard risk stratification systems that are used to prognosticate GISTs and guide the use of adjuvant systemic therapy are insufficient when used on tumors arising from rare primary sites, and, in the case of colon GISTs, this application may actually lead to detrimental effects on patient outcome and survival.
The surgical approach to GISTs varies based on organ of origin. Our study shows that rectal GISTs were less likely to undergo primary resection compared to gastric and colon GISTs (71.8% vs. 82.1% vs. 83.7% respectively, P<0.001), which was consistent when stratified by tumor size except for tumors less than 2 cm. Previous work using the SEER database has demonstrated that there is no significant difference in GIST-specific mortality in patients with <2 cm GISTs who undergo surgical resection versus those who do not (19). Furthermore, we also show that surgical treatment of patients with rectal GIST, in contradistinction to colonic GIST, is more likely to be local excision (51.1% vs. 8.4%, P<0.0001). This is partly explained by the advances in transanal endoscopic surgical approaches by gastrointestinal surgeons where as these are either technically not possible (colon) or not frequently performed (gastric) by a majority of gastrointestinal surgeons. Notably, rectal GISTs were found to have a lower rate of R0 or R1 resection than gastric or colon GISTs (77.7% vs. 90.5% vs. 83.5%, P=0.018).
Taken together, our study reinforces the notion that the underlying biology of GISTs is different across primary sites, which can translate to differences in response to systemic therapy and/or improvements in overall survival. The rarity of these particular tumor types has been an impediment to conducting site-specific investigational studies. Many surgical series and randomized control trials report very few primary colorectal GISTs, and therefore oncologic guidelines address the histologic subtype without consideration to the primary site (7,11). However, our data suggests that not all GISTs behave the same and therefore warrant continued research and individualized therapy.
While the utilization of a large national database has allowed us to assemble the largest study cohort of colorectal GISTs to date, there are still limitations. Most importantly, there are a number of prognostic factors for GISTs that are either not reported in the NCDB or very inconsistently reported. While number of mitoses per high-power field, KIT immunohistochemistry, KIT mutations, PDGFR mutations, and tumor multiplicity have more recently started to be collected in the NCDB, we found that there was an unacceptably high number of cases without these parameters being reported (>50%) and therefore did not include them as covariates. These are likely to be significant factors in understanding the suggested difference in tumor biology and response to systemic therapy, especially if colon GISTs do not demonstrate improvement in overall survival with the use of adjuvant tyrosine kinase inhibitors. Furthermore, GISTs are inconsistently reported to the NCDB due to strict reportability standards. For GISTs to be included in state tumor registries, they must be read pathologically as “malignant” or exhibit strict clinical features that demonstrate malignancy, such as multiple foci, lymph node involvement, metastases, or the administration of systemic therapy. Clinically, however, we do not classify GISTs as benign or malignant and instead stratify their risk by tumor grade and mitotic rate, as in the NIH-Fletcher system (13). Therefore, there is a concern that, not only are GISTs underreported, but there is a bias towards more aggressive GISTs in the NCDB (20). It is unclear if this bias is differently applied based on primary site. Finally, we are unable to assure that the systemic therapy utilized in this analysis represents the standard of care targeted therapy, imatinib or sunitinib or regorafenib, although use of any of these drugs would be captured in the NCDB as “single-agent chemotherapy”. A more comprehensive tracking system to monitor specific patient-level type of systemic therapy is needed to examine effectiveness of specific targeted therapy such as the use of tyrosine kinase inhibitors in these tumors.
Conclusions
In summary, in this largest descriptive study of colon and rectal GISTs to date, we have found that patients with rectal GISTs receive systemic therapy and multi-modality therapy at a higher frequency compared with both colon and gastric GISTs, and they also have the best overall survival. Individuals with colon GISTs, while receiving systemic therapy at frequencies comparable to gastric GISTs, have worse overall survival than both rectal and gastric GISTs, do not show an improvement in overall survival with adjuvant systemic therapy, and actually show worse overall survival with adjuvant systemic therapy in small <5 cm tumors. This suggests that tumor biology is different between the primary sites and highlights the continued need for investigational studies dedicated to colon and rectal GISTs to optimize clinical outcome.
Acknowledgements
This work was supported by the Lampman Research Fund in Yale Surgical Oncology and the Yale University School of Medicine Medical Research Fellowship.
Funding: This publication was made possible by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed by the Yale Institutional Review Board (No. 00000596) and deemed exempt from review as a secondary data analysis.
References
- Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 2008;3:557-86. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 2014;27 Suppl 1:S1-16. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 2004;22:3813-25. [Crossref] [PubMed]
- Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol 1999;23:82-7. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898-905. [Crossref] [PubMed]
- Parsons LS, editor. Performing a 1:N case-control match on propensity score. Proceedings of the 29th Annual SAS Users Group International Conference. Montreal, Canada: SAS Institute, 2004.
- Lee YC, Lee YL, Chuang JP, et al. Differences in survival between colon and rectal cancer from SEER data. PloS one 2013;8:e78709. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Kukar M, Kapil A, Papenfuss W, et al. Gastrointestinal stromal tumors (GISTs) at uncommon locations: a large population based analysis. J Surg Oncol 2015;111:696-701. [Crossref] [PubMed]
- Coe TM, Fero KE, Fanta PT, et al. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J Gastrointest Surg 2016;20:1132-40. [Crossref] [PubMed]
- Bilimoria KY, Wayne JD, Merkow RP, et al. Incorporation of adjuvant therapy into the multimodality management of gastrointestinal stromal tumors of the stomach in the United States. Ann Surg Oncol 2012;19:184-91. [Crossref] [PubMed]