A phase 2 trial of personalized cytotoxic therapy based on tumor immunohistochemistry in previously treated metastatic pancreatic cancer patients
Introduction
Metastatic pancreatic adenocarcinoma (mPC) continues to have a poor outcome despite recent advances with systemic chemotherapy. Gemcitabine until recently was widely used as a single agent to treat patients with mPC and results in a median survival of approximately 6 months and 1-year survival of 20% (1). Recently two new regimens have been developed, a combination of 5-FU, leucovorin, oxaliplatin, and irinotecan commonly called FOLFIRINOX and nab-paclitaxel combined with gemcitabine (2,3). Selected patients with a good performance status treated with either of these regimens have a median survival of about 8–11 months and a one-year survival of 30–35% (2,3). Until recently, there was no established or approved regimen for second line therapy (4,5). Following progression after first line therapy some patients with mPC will have a rapid decline in performance status, but 30–50% are candidates for subsequent therapy (4-7). Numerous phase II studies have been reported in the literature with median survival times for this population of 2–5 months, and <5% surviving for more than a year (8-10). The combination of 5-FU and oxaliplatin (OFF or FOLFOX) as second line therapy after treatment with gemcitabine has been evaluated in two randomized studies but has marginal activity (11,12). The combination of 5-FU with a nanoliposomal formulation of irinotecan (MM-398) has emerged as a new standard 2nd line regimen after treatment with a gemcitabine or a gemcitabine based combination regimen. In this study (NAPOLI-1), 417 patients were randomized to either 5-FU/MM398, monotherapy with MM398 or 5-FU alone. The median overall survival (OS) in patients receiving 5-FU/MM398 was superior, 6.1 months (95% CI, 4.8–8.9) compared to 4.2 months (95% CI, 3.3–5.3) in the 5-FU arm (HR: 0.67; 95% CI, 0.49–0.92; P=0.012) (13).
The choice of a treatment regimen for mPC remains empiric and is at the discretion of the physician and patient. Molecular profiling (MP) of tumors is now widely available from commercial vendors using multiple platforms including immunohistochemistry (IHC), gene expression microarray (MA), FISH, and next generation sequencing (14). Using one or more of these assays, most tumors will have an “actionable target” for which a chemotherapeutic agent can be selected as therapy (15-17). The first study to be published in patients who were treated by an MP based selection provides support for continued investigation of this approach. In that study 66 patients with various advanced refractory solid tumors underwent a biopsy of a metastatic lesion and targets were identified using a Clinical Laboratory Improvement Amendment (CLIA)-validated IHC assays. Commercially available agents were prescribed based on the MP. The study met its primary endpoint with 27% of patients having a longer progression-free survival (PFS) with the MP suggested regimen compared to the PFS on the last unselected prior therapy before entering the study (16). The response rate was 10% and the treating physician’s empiric choice of the next treatment regimen without the availability of MP was very different, suggesting the clinical utility of this approach (18). The phase II study presented here was designed to evaluate the benefits of IHC based therapy in a cohort of mPC patients who had progressed ≥1 prior therapy with 1-year survival as the primary endpoint.
Methods
Eligibility criteria
Eligible patients (≥18 years of age) had mPC and had received ≥1 prior therapy for the treatment of metastatic disease. One or more metastatic tumors had to be measurable by computed tomography (CT) scan and accessible for a tumor biopsy. Other pertinent eligibility criteria were acceptable bone marrow, kidney and liver function, prior therapy must have been completed ≥3 weeks before starting study and side effects of prior therapy must have resolved to ≤ grade 1. A Karnofsky performance status (KPS) ≥70 was required. All patients were required to sign an institutional review board approved consent form prior to participating in any study-related activities.
MP of tumors
Patients who met eligibility criteria underwent a percutaneous biopsy using an 18-gauge needle with at least three passes. The tumor sample was divided into three cores after keeping one for pathological confirmation of malignancy and then flash frozen and shipped on dry ice to the destinations below. Priority was given for IHC for its superior ability to visually assess the presence of a biomarker in targeted cell population (cancer cells), and was performed at a CLIA certified laboratory by experienced board-certified pathologists (Caris Life Sciences, Phoenix, AZ, USA) where up to 17 markers were assayed (representative IHC data is presented for the interested reader as Table S1). One of the other two samples was sent to the Translational Genomics Research Institute (TGen, Phoenix, AZ, USA) for flow sorting and array comparative genomic hybridization (aCGH). The other sample was sent to the Johns Hopkins University (Baltimore, MD, USA) for MA analysis.

Full table
Selection of therapy
The study broadly followed previously published guidelines in a pilot study of MP in refractory solid tumors (16). Only commercially available agents were recommended for therapy. In cases of combination therapy, only published regimens could be prescribed. The IHC results were expected within ten business days from date of biopsy. A tumor board consisting of the study investigators and the treating oncologist met weekly. Treatment recommendations were made on the basis of the CLIA certified IHC assay. The tumor board (which included RK Ramanathan, MT Barrett, R Posner, NV Rajeshkumar, M Aziz, EC Stites, WS Hlavacek, M Hidalgo, DD Von Hoff) reviewed the patient’s prior therapy and other relevant clinical factors such as performance status, comorbidities, and organ function. Publications for treatment regimens were also reviewed and discussed. Therapy was selected using the following principles: (I) combination therapy had preference over single agent if there was no contraindication; (II) avoid prior agents that patient had received; (III) if more than one agent or agents are options, then the toxicity profile and efficacy was taken into consideration. Based on the tumor board recommendations the principal investigator (RK Ramanathan) approved the treatment recommendation including a published reference which was communicated in writing to the treating physician. If no suitable standard agents were identified on MP, then the patient could be treated at the physician’s discretion.
Response and toxicity assessments
The treating physician followed published guidelines for follow-up, assessments, and dose modification. Serious adverse events were evaluated at every patient visit. Patients were evaluated for tumor response every 8 weeks by RECIST 1.1 criteria (19).
MP
A percutaneous computed tomography (CT) or ultrasound (US)-guided biopsy was performed of an accessible metastatic lesion.
IHC assays
Formalin-fixed paraffin-embedded tumor samples were analyzed with up to 17 commercially available antibodies against protein biomarkers using automated staining techniques (Benchmark XT, Ventana, Tucson, AZ, USA; and AutostainerLink 48, Dako, Carpinteria, CA, USA).
HER2/CEP17 fluorescent in-situ hybridization (FISH) assay
FISH was used for evaluation of the HER2 [HER2/CEP17 (chromosome 17 centromere) probe, Abbott Molecular/Vysis, Abbott Park, IL, USA]. HER2/CEP17 ratio ≥2.2 was considered amplified [based on guidelines from the College of American Pathology (CAP)/American Society of Clinical Oncologists (ASCO) 2007].
Sanger sequencing
Mutation analysis using Sanger sequencing of selected regions of KRAS was performed using M13-linked polymerase chain reaction (PCR) primers designed to amplify targeted sequences. PCR products were bidirectionally sequenced using the BigDye Terminator v1.1 chemistry (Applied Biosystems, Grand Island, NY, USA), and analyzed using the 3,730 DNA Analyzer (Applied Biosystems). Sequence traces were analyzed using Mutation Surveyor software v3.25 (Soft Genetics, San Francisco, CA, USA).
Flow cytometry and aCGH
Biopsies were thawed then minced in the presence of NST buffer and DAPI according to published protocols (20). Nuclei were disaggregated then filtered through a 40 µm mesh prior to flow sorting with an Influx cytometer (Becton-Dickinson, San Jose, CA, USA) with ultraviolet excitation and DAPI emission collected at >450 nm. DNA content and cell cycle were analyzed using the software program MultiCycle (Phoenix Flow Systems, San Diego, CA, USA). DNA was extracted from each sorted sample using Qiagen micro kits (Qiagen Valencia, CA, USA). For each hybridization 100 ng of genomic DNA from each sample and of pooled commercial 46XX reference (Promega) were amplified using the GenomiPhi amplification kit (G.E. Healthcare, Piscataway, NJ, USA). Subsequently, 1 µg of amplified sample and 1µg of amplified reference template were digested with DNaseI then labeled with Cy-5 dUTP and Cy-3 dUTP respectively, using a BioPrime labeling kit (Invitrogen, Carlsbad, CA, USA). All labeling reactions were assessed using a Nanodrop assay (Nanodrop, Wilmington, DE, USA) prior to mixing and hybridization to CGH arrays with 400,000 oligonucleotide features (Agilent Technologies, Santa Clara, CA, USA). After hybridization, all aCGH slides were scanned using an Agilent 2565C DNA MA scanner. Images were analyzed using Agilent Feature Extraction software version 10.7 (FE 10.7) with default settings. These data have been deposited in the GEO database under the accession number GSE64462. All aCGH experiments were evaluated using a series of quality control (QC) metrics. These include background noise, signal intensities, and signal to noise ratios for each dye-specific channel, the reproducibility of a series of replicate control probes on the arrays, and a measure of the spread of the distribution of the log2 ratios reported in each experiment. The data from arrays were analyzed in Genome Workbench using an aberration detection algorithm (ADM2) to define and rank all amplicons and deletions (21).
MA
MA was performed using the Affymetrix GeneChip® (Santa Clara, CA, USA; data not shown).
Statistical considerations
This was an open label phase II study. The primary objective was to determine the 1-year survival from start of registration for patients who received at least one dose of therapy. The goal was to improve the 1-year survival of patients receiving 2nd line or more salvage therapy from the historical rate of 5% to ≥20% by means of IHC-based therapy. Assuming the number of patients alive at 1 year was binomially distributed, this design had a one-sided significance level, alpha, of 0.03 and a power of 86% for detecting a true survival rate of at least 20% versus the null hypothesis survival rate of 5% or less. If 5 or more patients of the 35 evaluable patients enrolled were alive at 1 year, this would be considered adequate evidence of promising activity. Secondary objectives were to determine the response rate (according to RECIST 1.1), PFS, and correlation of molecular profiles and outcomes. PFS were assessed using the method of Kaplan Meier. Cox regression was used to determine the effect of MP on survival. Because subjects received therapy with commercially available agents, toxicity data were not collected.
Results
Patient characteristics
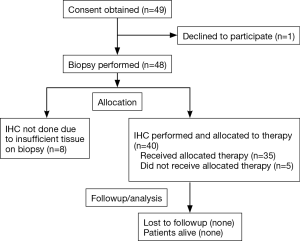
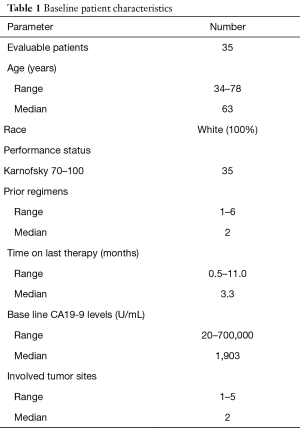
Forty-nine patients were accrued at the Virginia G. Piper Cancer Center, Scottsdale, AZ between September 2010 and January 2012. One patient withdrew consent prior to a biopsy, and 48 were scheduled and underwent a biopsy without any major complications (Figure 1). Thirteen patients did not start protocol therapy either due to insufficient tumor for analysis on biopsy (n=8) or due to worsening cancer related symptoms after biopsy which precluded further treatment (n=5). Table 1 describes demographics of evaluable patients (n=35), males (55%), the majority had KPS of 70–80 and age range is 34–78 years (median 63 years). Time from the first diagnosis of mPC to biopsy on this protocol ranged from 5.8–26.7 months (median 16.1 months). All patients had prior gemcitabine-based therapy and had a median of 2 prior regimens (range, 1–6). The time from patient signing consent to performing the biopsy was a median of 4 days (range, 1–9 days) and from biopsy to availability of the IHC report was a median of 13 days (range, 4–16 days).
Full table
IHC results
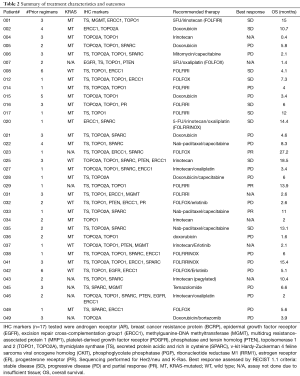
In the 40 patients who had adequate tissue for IHC analysis, the most common targets identified were overexpression of topoisomerases (TOPO) 1 (73%) and TOPO2A (63%), under-expression of thymidylate synthase (TS) (63%), overexpression of SPARC (48%), and under-expression of excision repair cross-complementation group 1 protein (ERCC1) (40%). Most patients had at least two IHC targets which were actionable (Table 2).
Full table
Mutation status
KRAS codon 12 mutations by sequencing were seen in 33 of the 38 tested samples (87%). When 5 samples which had tested initially negative for a KRAS mutation were resequenced using DNA from flow sorted purified samples, 3 additional Kras codon 12 mutations were identified with another one sample having an uncommon Q61H mutation.
aCGH
A total of 39 biopsies were processed with flow cytometry and aCGH. In seven cases there was no tumor detected whereas two additional cases failed QC metrics in the aCGH analysis. The 30 tumor samples that were successfully sorted and profiled included 24 patients who received treatment. The use of flow sorted samples enabled objective thresholds for the identification of gains and losses and the discrimination of homozygous loss (log2ratio <−3.0) and focal amplicons (<7.0 Mb and log2ratio >1.0) in each tumor. This high-resolution analysis identified multiple copy number aberrations in known drivers of pancreatic cancer and the genes targeted by the IHC assays (Table 3). The most frequent aberrations were copy number losses of 9p21.3 and 18q21.1. These included homozygous deletions targeting CDKN2A and SMAD4; two tumor suppressor genes with known roles in pancreatic cancer. Strikingly, we detected a novel homozygous deletion affecting RASA1, a negative regulator of wild type (WT) KRAS, in the one confirmed case that was KRAS WT.
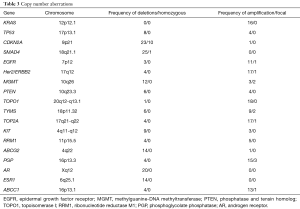
Full table
Treatment selection
Based on the IHC results, patients received therapy with commercially available single agents or combination therapy. Prior agents on which patients’ cancers had progression were avoided. Therapy was modified by the treating physician in four cases (Table 2). Patient #006 was prescribed capecitabine, in this case mitomycin was added to the regimen. Patient #020 was prescribed FOLFOX, but FOLFIRINOX was chosen for therapy. Patient #043 was prescribed irinotecan and received therapy on a phase I clinical trial with a pegylated form of irinotecan. Patient #049 had doxorubicin as the prescribed regimen and was treated with a combination of doxorubicin and bortezomib (22). Common treatments prescribed were FOLFIRI (n=31%), single agent irinotecan or other irinotecan combinations excluding fluorouracil (23%), FOLFOX (17%), doxorubicin (17%), erlotinib combinations (11%), and nab-paclitaxel based combination (9%).
Efficacy results
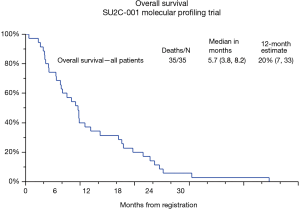
In the study population (n=35) there were 3 partial responses (PRs) (9%), these patients received FOLFOX (#023), FOLFIRI (#029), and nab-paclitaxel/capecitabine (#033); and 9 patients had stable disease (SD) for a disease control rate of 35% (95% CI, 19–54%) (Table 2). Time on study ranged from 0–8.6 months (median 1.3 months, 95% CI, 1–1.6 months). All patients have died, with survival ranging from 0.4 months to 26.8 months. The median PFS is 2.4 months (95% CI, 2.1–3.9 months) and median survival is 5.7 months (95% CI, 3.8–8.2 months). The 1-year survival rate was 20% (95% CI, 7–33%) for all 35 patients (Figure 2).
Discussion
This study illustrates the feasibility and challenges of a MP directed therapy in mPC patients following a first line therapy. At present a tumor biopsy consisting of adequate number of malignant tissue is necessary to construct a molecular profile using different technologies, In the patient population studied, we were able to do percutaneous biopsies safely and obtain tissue for IHC analysis in most cases. In all cases we were able to determine “actionable targets” in evaluable patients. It is interesting that based on the IHC results, irinotecan (TOPO1) and/or fluorouracil (TS) were common agents prescribed in the study. FOLFIRINOX is a standard first line regimen for mPC and the respective contributions of the individual therapeutic components (5FU, oxaliplatin, irinotecan) and tumor biomarkers (TOPO1, TS) to the efficacy of the regimen are not known. A number of pilot studies in mPC have suggested that FOLFIRI is active (23,24) and support for this hypothesis also comes from a recently completed study (NAPOLI-1), which established MM398 in combination with 5-FU as an effective 2nd line regimen (13). The therapeutic ratio of this combination could be enhanced if activity is increased in tumors with TOPO1 expression. Although doxorubicin was prescribed in 17% of patients based on MP, the efficacy results were disappointing. ATP-binding cassette transporter proteins mediate resistance to a number of chemotherapeutic agents (25) and in patients treated with doxorubicin, intrinsic resistance is suggested by overexpression of MDR1 and/or BRCP in all but one patient (#002), this patient had SD and had a survival of 10.7 months (Table 2). It is noteworthy that MP results ribonucleotide reductase M1 (RRM1) did not suggest gemcitabine as a treatment option for patients. All patients had prior gemcitabine before entering the study and further studies need to elucidate whether IHC markers accurately predict resistance to gemcitabine from prior exposure. KRAS mutations are seen in almost all pancreatic cancers, but still there are no treatment strategies for this mutated gene. Validation of molecular markers as a clinical tool continues to evolve. Since the study was completed, the validity of expression of the ERCC protein by the previously used batches of 8F1 antibody could not be replicated in non-small cell lung cancer and has been replaced by newer methodology (26). The usefulness of SPARC is also in question as a subset analysis of mPC patients treated with nab-paclitaxel and gemcitabine in the randomized MPACT trial did not show a benefit of treatment for SPARC overexpression (27).
Additional MP was performed, with aCGH in 24 treated patients. In addition to aberrations targeting known drivers of pancreatic cancer, we identified unique and recurring aberrations in each tumor genome (Table 3). These included high level focal amplicons targeting oncogenes (MYC, AURKC, FGFR1) and homozygous deletions of tumor suppressor genes (MAP2K4, L3MBTL4). In many cases the targeted genes are associated with specific pancreatic cancer hallmarks, including KRAS signaling (focal amplification of FNTA and homozygous deletion of RASA1) and DNA repair (focal amplification of USP47). Of significant interest are those aberrations targeting genes and pathways (e.g., AURKC, FGFR1, AKT) that may be exploited in future studies for therapeutic targeting. MA was also performed in selected samples (n=27) and the expression profile was matched to the chemosensitivity profiles of the NCI 60 cell line and an institutional database of patient derived pancreatic xenografts (28) (data not shown). However, results did not correlate to the treatment selection by IHC in this study. This approach is still worthwhile, and requires further study as pharmacogenomics modeling based on the expression profile of circulating tumor cells from mPC patients appears to predict response and outcomes to cytotoxic therapy (29).
This study is the first to prospectively biopsy tumor and treat mPC patients according to the IHC results. The study endpoint of 1-year survival of ≥20% was met and several new treatment regimens for pancreatic cancer such as irinotecan, FOLFIRI, nab-paclitaxel/capecitabine (30) have been identified as promising leads, and require validation in subsequent studies. Our study has limitations of a single arm study and has potential for selection bias. In fact, the median time from diagnosis of mPC to enter the study was surprisingly 16.1 months, which is considerably more than the expected survival of 9–12 months and these patients had a median of two prior regimens. Still a response rate of 9% and disease control rate of 34% in these heavily pretreated patients argues for some benefit of this approach. aCGH and MA were performed in this study, but were not used to make treatment decisions. Ongoing work will attempt to integrate global information into actionable pathways that can provide information for therapy (31,32). We believe it is time for randomized studies to be conducted. All pancreatic patients receive cytotoxic therapy and IHC markers, especially RRM1, ERCC, TS and TOPO1 appear to predict sensitivity in different tumor types to gemcitabine, platinum, 5-FU, and irinotecan; respectively. In mPC, a study design can stratify patients to first line therapy to gemcitabine/nab-paclitaxel or FOLFIRINOX based on these IHC markers. Similarly, in a 2nd line setting stratification can be made to gemcitabine, 5-FU, FOLFOX, FOLFIRI (or MM-398).
The use of MP assays has been increasing in clinical practice with availability of a number of different platforms. Retrospective MP results have been published in a number of different tumor types showing actionable mutations/deletions or amplifications in a number of genes (14,30). Clinical trials have been initiated. A recently reported study enrolled 25 evaluable metastatic breast cancer patients following ≥3 lines of therapy. Treatment selection was made by biopsy of an accessible lesion and “multi-omic” MP. The study met its primary endpoint with PRs noted in 5/25 (20%) and SD in 8/25 (32%) of patients (17). Thus, data is accumulating on the feasibility and usefulness of MP based therapy. In the next decade we should expect validation of increasingly sophisticated “pan-omic” assays for MP based therapy with resulting benefits to patients and improved outcomes.
Acknowledgements
Funding: Supported by a Stand Up to Cancer (SU2C-AACR) Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0509).
Footnote
Conflicts of Interest: This work was presented in part at the American Association for Cancer Research; 103rd Annual Meeting (LB-221 and A 3697), 2012, Chicago, IL, USA. RK Ramanathan: meeting honorarium and travel expenses from Caris Life Sciences; DD Von Hoff: consultant and stock options, Caris Life Sciences; Z Gatalica, J Xiu: employment and stock options, Caris Life Sciences. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of Honor Health Research Institute/TGEN (No. WIRB 20101207) and informed consent was taken from all the patients.
References
- Sohal DP, Mangu PB, Khorana AA, et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2784-96. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Petrelli F, Borgonovo K, Ghilardi M, et al. What else in gemcitabine-pretreated advanced pancreatic cancer? An update of second line therapies. Rev Recent Clin Trials 2010;5:43-56. [Crossref] [PubMed]
- Aprile G, Negri FV, Giuliani F, et al. Second-line chemotherapy for advanced pancreatic cancer: Which is the best option? Crit Rev Oncol Hematol 2017;115:1-12. [Crossref] [PubMed]
- Mancuso A, Sacchetta S, Saletti PC, et al. Clinical and molecular determinants of survival in pancreatic cancer patients treated with second-line chemotherapy: results of an Italian/Swiss multicenter survey. Anticancer Res 2010;30:4289-95. [PubMed]
- Altwegg R, Ychou M, Guillaumon V, et al. Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer. World J Gastroenterol 2012;18:1357-64. [Crossref] [PubMed]
- Brell JM, Matin K, Evans T, et al. Phase II study of docetaxel and gefitinib as second-line therapy in gemcitabine pretreated patients with advanced pancreatic cancer. Oncology 2009;76:270-4. [Crossref] [PubMed]
- Hosein PJ, de Lima Lopes G Jr, Pastorini VH, et al. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am J Clin Oncol 2013;36:151-6. [Crossref] [PubMed]
- Dakik HK, Moskovic DJ, Carlson PJ, et al. The use of GTX as second-line and later chemotherapy for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol 2012;69:425-30. [Crossref] [PubMed]
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;32:2423-9. [Crossref] [PubMed]
- Gill S, Ko YJ, Cripps C, et al. PANCREOX: A Randomized Phase III Study of 5-Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol 2016;32:3914-20. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- My Cancer Genome. Available online: https://www.mycancergenome.org/content/molecular-medicine/types-of-molecular-tumor-testing. Accessed July 17, 2017.
- Von Hoff DD, Penny R, Shack S, et al. Frequency of potential therapeutic targets identified by immunohistochemistry (IHC) and DNA microarray (DMA) in tumors from patients who have progressed on multiple therapeutic agents. J Clin Oncol 2006;24:abstr 3071.
- Miller VA, Ross JS, Wang K, et al. Use of next-generation sequencing (NGS) to identify actionable genomic alteractions (GA) in diverse solid tumor types: The Foundation Medicine (FMI) experience with 2,200+ clinical samples. J Clin Oncol 2013;31:abstr 11020.
- Jameson GS, Petricoin EF, Sachdev J, et al. A pilot study utilizing multi-omic molecular profiling to find potential targets and select individualized treatments for patients with previously treated metastatic breast cancer. Breast Cancer Res Treat 2014;147:579-88. [Crossref] [PubMed]
- Von Hoff DD, Stephenson JJ Jr, Rosen P, et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol 2010;28:4877-83. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Holley T, Lenkiewicz E, Evers L, et al. Deep clonal profiling of formalin fixed paraffin embedded clinical samples. PLoS One 2012;7:e50586. [Crossref] [PubMed]
- Lipson D, Aumann Y, Ben-Dor A, et al. Efficient calculation of interval scores for DNA copy number data analysis. J Comput Biol 2006;13:215-28. [Crossref] [PubMed]
- Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 2003;9:1136-44. [PubMed]
- Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009;101:1658-63. [Crossref] [PubMed]
- Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012;69:1641-5. [Crossref] [PubMed]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002;2:48-58. [Crossref] [PubMed]
- Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 2013;368:1101-10. [Crossref] [PubMed]
- Hidalgo M, Plaza C, Musteanu M, et al. SPARC Expression Did Not Predict Efficacy of nab-Paclitaxel plus Gemcitabine or Gemcitabine Alone for Metastatic Pancreatic Cancer in an Exploratory Analysis of the Phase III MPACT Trial. Clin Cancer Res 2015;21:4811-8. [Crossref] [PubMed]
- Hidalgo M, Bruckheimer E, Rajeshkumar NV, et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther 2011;10:1311-6. [Crossref] [PubMed]
- Yu KH, Ricigliano M, Hidalgo M, et al. Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer. Clin Cancer Res 2014;20:5281-9. [Crossref] [PubMed]
- Somer BG, Schwartzberg LS, Arena F, et al. Phase II trial of nab-paclitaxel (nanoparticle albumin-bound paclitaxel (ABX) + capecitabine (XEL) in first-line treatment of metastatic breast cancer (MBC). J Clin Oncol 2007;25:abstr 1053.
- Gatalica Z, Millis S, Chen S, et al. Integrating molecular profiling into cancer treatment decision making: Experience with over 35,000 cases. J Clin Oncol 2013;31:abstr 11001.
- Barrett MT, Deiotte R, Lenkiewicz E, et al. Clinical study of genomic drivers in pancreatic ductal adenocarcinoma. Br J Cancer 2017;117:572-82. [Crossref] [PubMed]
- Hu YC, Komorowski RA, Graewin S, et al. Thymidylate synthase expression predicts the response to 5-fluorouracil-based adjuvant therapy in pancreatic cancer. Clin Cancer Res 2003;9:4165-71. [PubMed]
- Qiu LX, Tang QY, Bai JL, et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer 2008;123:2384-9. [Crossref] [PubMed]
- Lee SJ, Choi YL, Park YH, et al. Thymidylate synthase and thymidine phosphorylase as predictive markers of capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemother Pharmacol 2011;68:743-51. [Crossref] [PubMed]
- Li P, Fang YJ, Li F, et al. ERCC1, defective mismatch repair status as predictive biomarkers of survival for stage III colon cancer patients receiving oxaliplatin-based adjuvant chemotherapy. Br J Cancer 2013;108:1238-44. [Crossref] [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Braun MS, Richman SD, Quirke P, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 2008;26:2690-8. [Crossref]
- Rodrigo RS, Nathalie A, Elodie T, et al. Topoisomerase II-alpha protein expression and histological response following doxorubicin-based induction chemotherapy predict survival of locally advanced soft tissues sarcomas. Eur J Cancer 2011;47:1319-27. [Crossref] [PubMed]
- O'Malley FP, Chia S, Tu D, et al. Topoisomerase II alpha protein and responsiveness of breast cancer to adjuvant chemotherapy with CEF compared to CMF in the NCIC CTG randomized MA.5 adjuvant trial. Breast Cancer Res Treat 2011;128:401-9. [Crossref] [PubMed]