Investigation of targetable predictive and prognostic markers in gallbladder carcinoma
Introduction
Gallbladder carcinoma shows marked geographic variability in incidence and as per the Globocan data it is the 20th most common cancer in the world, with an estimated 178,100 new cases diagnosed in 2012 (1). Geographic areas that report a high incidence and high mortality rates include South Asia (Northern India, Pakistan, Bangladesh, and Nepal), South America (Chile, Bolivia, and Peru), and East Asia (Japan, Korea, and China) (1). Due to its low incidence in western countries gallbladder cancer has been poorly characterized leading to incomplete data on the pathologic evaluation, molecular markers and targeted therapy options.
Genetic profile of hepatobiliary cancers has been infrequently studied. In a review of the molecular profiling of biliary tract cancers, Jain and Javle have highlighted variation in the genomic landscape based on the tumor location, extrahepatic vs. intrahepatic and gallbladder. Actionable mutations are present in gallbladder cancers and vary in different patient populations (west vs. east) (2). In view of this variation it appears relevant to obtain molecular profiles of predictive and prognostic markers from regions with a high incidence of gall bladder cancer and hence this study was planned in a substantial study sample in the Indian population.
A multitude of molecular biomarkers including epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and human epidermal growth factor receptor 2 (HER2/Neu) have been considered as potential therapeutic targets in gallbladder cancer. A large variety of downstream pathways, such as RAS, RAF, MEK-ERK1/2 and PI3k-AKT-mTOR, are involved (3,4). VEGF plays a significant role in neo-angiogenesis of tumors providing tumor tissue with nutrition and a metastatic potential. Endothelial cell proliferation is mediated through binding of VEGF and VEGF receptors (5). HER2/Neu and p53 seem to play a critical role in tumor initiation and progression of gallbladder carcinogenesis. HER2/Neu, EGFR and VEGF are targeted by trastuzumab in breast cancer, gefitinib and erlotinib in non-small cell lung cancer, and bevacizumab in colorectal cancer respectively (5,6). SWOG S0809 a Phase II Intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma has shown well tolerated and promising efficacy. Limited genome profiling studies have been done in gallbladder cancers. High profiling techniques in biliary tract malignancies have identified alterations in the MAP kinase pathway, EGFR, FGF pathway, PI3K pathway (7). In the current study we have quantified the expression of HER2/Neu, p53, EGFR and VEGF proteins to identify subgroup of targetable carcinomas and also applied a screening for 50 hotspot oncogene panel in next generation sequencing in representative tumor samples.
Methods
This study was conducted at Dr. Ram Manohar Lohia Institute of Medical Sciences Lucknow and King George’s Medical University, Lucknow, India. Approvals were obtained from the institutional ethical committee (IEC) of the authors’ institutions.
Study sample
A large cohort of 268 histologically diagnosed cases of gallbladder carcinoma between Jan 2012 to Dec 2016, including specimen of radical cholecystectomy (24.3%), simple cholecystectomy (14.9%), gallbladder biopsy (42.5%) and biopsy from metastatic sites including omentum (3%), liver (4.5%), lymph node (3.7%), scar site (5.2%) and distant metastasis (1.9%) were included in the study.
Clinicopathological characteristics were obtained from medical records of the patients. Tumor staging has been done according to TNM staging system (AJCC, eight edition, 2017) (8).
Histopathological & immunohistochemical analyses
All diagnostic blocks and slides were reviewed by two pathologists (Azfar Neyaz, Nuzhat Husain) and histological sub-typing and grading was done according to WHO classification (9) (Table 1). Morphologically distinct areas of each tumor were identified and reported independently for each marker expression.
Full table
Immunohistochemical analysis of selected markers for the study was performed on paraffin-embedded tissue sections. Tissue sections of 5 µm were deparaffinized in xylene and then re-hydrated with sequential washes of 100%, 70%, and 50% ethanol. Endogenous peroxidase activity was inhibited with 3% hydrogen peroxidase (Loba Chemie, India) in methanol for 30 minutes. For antigen retrieval, slides were placed in 50 mM citrate buffer pH 6.0 to unmask the epitopes at 95 °C. Tissue sections were then incubated with specific antibodies for 1 hour at room temperature. The antibodies used included HER2 (Ventana, anti-HER2/Neu 4B5 Rabbit Monoclonal Primary Antibody, USA), EGFR (Biogenex Cat No. AN473-5ME, USA), VEGF (Biogenex Cat No. AR483-5R, USA) and p53 (Dako, Cat No. IS6130, Denmark). Slides were rinsed with Tris buffer (pH 7.4) for three times followed by treatment with polymer based secondary antibody kit with 3′3 diaminobenzidine tetra hydrochloride (DAB), as substrate (Dako REALTM EnVisionTM Detection System Peroxidase/DAB+* Rabbit/Mouse, Ref. K5007). All sections were counterstained with 0.1% Hematoxylin. After dehydration tissue sections were fixed with permanent mounting medium and covered with glass cover slips. Positive and negative controls were included in each run. All immunohistochemical stains were independently analyzed by two of the authors (Azfar Neyaz, Nuzhat Husain) and the immunohistochemical characteristics of each morphologically distinct area of the tumors were evaluated.
Criteria for positive immunohistochemical staining
HER2/Neu status was assessed following the American Society of Clinical Oncology/College of American Pathologists guidelines for gastric and gastroesophageal junction cancer. The cases were considered as positive, if >10% tumor cells in large specimen or at least 5 cohesive cells in biopsy, showed complete or basolateral membranous staining with 3+ intensity. Cases were considered as equivocal, if >10% tumor cells in large specimen or at least 5 cohesive cells in biopsy showed moderate/weak complete or basolateral membranous staining and negative if there were absent to weak staining in only one part of membrane (10). For EGFR and VEGF, the percentage of tumor cells within a given component was recorded. EGFR over expression was considered as >10% complete membranous reactivity with 3+ intensity (11). Positive interpretation for VEGF was considered at ≥30% cytoplasmic and/or membranous reactivity with intensity of 2+/3+ (11). p53 nuclear reactivity in ≥50% tumor cells with a 2+/3+ intensity or complete negative staining was considered as a phenotype of in mutant gene expression (12). Discordant results were resolved on a multi-headed microscope.
Tissue microarray (TMA) and HER2/Neu fluorescent in situ hybridization (FISH)
Representative areas from the tumor were marked on the slide and then on the corresponding block. For each case, 2 to 3 tumor tissue cores (1.5–2.0 mm diameter) from representative tumor areas were punched out and embedded into a new paraffin array block using an automated TMA (TMA Grandmaster, company, USA).
FISH analysis was performed on deparaffinized 4 µm tissue sections using the ZytoLight FISH Tissue Implementation Kit (Cat Z2028-20, Bio SB, USA). Briefly, TMA sections were incubated over night at 56 °C. Slides were deparaffinized by incubation of slides at 70 °C for 10 min followed by 2 washes in xylene for 10 min. Sections were rehydrated in decreasing concentration of ethanol and washed 2 times in deionized water for 2 min. The slides were then incubated in pre-warmed heat pre-treatment solution citric at 98 °C for 15 min, and immediately washed 2 times in deionized water for 2 min. Proteins were digested by addition of pepsin solution for 10 min at 37 °C followed by one wash in buffer SSC for 5 min and in deionized water for 1 min. Dehydration of section were done through 70%, 90%, and 100% ethanol for 1 min each, slides were allowed to air dry. 10 µL of ZytoLight HER2 DNA probe (ZytoLight Spec ERBB2/CEN17 Dual colour probe kit, USA) was applied to the sections, cover slipped and sealed with glue. Sections were denatured at 75 °C for 10 min, followed by incubation for 16 hours in ThermoBrite FISH Slide Denaturation and Hybridization system (Fisher Scientific, USA) at 37 °C. Post incubation cover slip and glue were removed and slides were washed once with prewarmed (37 °C) wash buffer followed by dehydration in graded ethanol. Slides were air dried and counter stained with 10 µL of DAPI (diamidino-phenyl-indole), cover slipped and sealed with nail polish. Finally, the slides were observed under fluorescence microscope (Zeiss.axioimager Z2, Germany). Interpretation and scoring was done as positive, equivocal and negative. Cases were scored as positive if HER2/CEN17 FISH signal ratio was ≥2 or HER2/CEN17 FISH signal ratio was <2 but average of HER2 gene copy number was >6 signal/nucleus. Cases were scored as equivocal if HER2/CEN17 FISH signal ratio is <2 with average HER2 gene copy number >4 and <6 signals/nucleus and cases were scored as negative if HER2/CEN17 FISH signal ratio was <2 with average HER2 gene copy number <4 signals/nucleus. For analysis, at least 50 non-overlapping, interphase nuclei of morphologically unequivocal neoplastic cells were analyzed.
DNA isolation from FFPE tissue
DNA from paraffin embedded sections was isolated by FFPE tissue DNA kit (Qiagen, USA). Briefly, 7–8 sections of 5–8 µ thickness were cut and dissolved in 1 mL xylene and the pellet was washed with 1 ml ethanol. After evaporation of excess ethanol, pellet was resuspended in 180 µL Buffer ATL and 20 µL proteinase K followed by incubation at 56 °C for 1 hour and at 90 °C for 1 hour. After incubation 200 µL Buffer and 200 µL of 100% ethanol was added. Suspension was transferred to column tube and centrifuged followed by washing with wash buffer 1 and 2. Finally DNA was eluted in elution buffer.
Next generation sequencing
The Ion Torrent Library was prepared using the Ion AmpliSeq cancer hotspot panel V2 (Thermo Fisher Scientific) sequencing assay targeting approximately 2,800 COSMIC mutations from 50 oncogenes and tumor suppressor genes. The Cancer Panel V2 assay detects mutations on selected hotspot regions of the following 50 genes: ABL1, AKT1, ALK, APC, ATM, BRAF, CDH1, CDKN2A, CSF1R, CTNNB1, EGFR, ERBB2, ERBB4, EZF2, FBXW7, FGFR1, FGFR2, FGFR3, FLT3, GNA11, GNAQ, GNAS, HNF1A, HRAS, IDH1, IDH2, JAK2, JAK3, KDR, KIT, KRAS, MET, MLH1, MPL, NOTCH1, NPM1, NRAS, PDGFRA, PIK3CA, PTEN, PTPN11, RB1, RET, SMAD4, SMARCB1, SMO, SRC, STK11, TP53, and VHL including 2,855 hotspots.
Extracted DNA was first quantified by a Picogreen protocol (Life Technologies, Carlsbad, CA, USA). Samples were barcoded using IonXpress Barcode Adapters (Life Technologies) to allow for discrimination between samples within a NGS run. The DNA concentration of the samples within one sequencing run was normalized using the Qubit 2.0 fluorometer (Thermo Fisher Scientific, USA) or the Ion Library Equalizer kit. The Ion AmpliSeq Library Kit 2.0 (Life Technologies) was used for library preparation. The library was mixed with Ion Sphere Particles (ISPs) and the subsequent emulsion PCR and enrichment were performed using the Ion PGMTM Template OT2 200 Template Kit and the Ion One Touch 2 instrument (Life Technologies). Sequencing was performed using the Ion PGMTM Sequencing 200 kit v2 using the Ion 316TM chip (Life Technologies) (maximum number of samples on 316 chip were 6). Twelve samples including two cases each of papillary adenocarcinoma, adenosquamous carcinoma, poorly differentiated adenocarcinoma, well differentiated adenocarcinoma and mucinous adenocarcinoma and two controls from cases of chronic cholecystitis were run on the Ion Torrent PGM System TM (Life Technologies) as described by the manufacturer.
Data analysis
The raw data was analyzed using the torrent suite software v3.6.2 (Life technologies). The coverage analysis was performed using the coverage analysis plug-in v3.6. Cases for which the number of mapped reads was <100,000 and/or the average base coverage was <500× were considered as non-informative. Mutations were detected using the Variant Caller plug-in v3.6 with low stringency settings (Life Technologies). As tumor specimens were admixed with normal tissue, a minimum coverage of 500× with at least 1% frequency was used as cutoff for a variant to be considered true.
Statistical analysis
Data has been presented as mean ± SD or median with range for continuous variables and as absolute and relative frequencies for categorical variables. Statistical analysis of the data was done on SPSS computer statistics programme (20.0.0.0, 2015). To determine the association between two or more than two variables, chi square test has been applied. All statistics were performed using 2-sided analysis, with a significance level of P<0.05.
Results
Patient characteristics of the study group
The study group comprised of 268 cases of gallbladder carcinoma with a mean age 49.5 years ranging from 25 to 80 years. Women comprised 77.6% of the study group with a mean (SD) age of 49.05 (9.80) years, and 22.4% were men with a mean (SD) age of 51.38 (10.42) years. Female-to-male ratio was 3.5:1 (Table 1). Regional spread of disease and distant metastasis did not show significant gender variation. This study included 65 cases that have undergone radical cholecystectomy with lymph node dissection and 40 (14.9%) cases with simple cholecystectomy. There was no significant difference in age at which both men and women underwent cholecystectomy. In 105 radical and simple cholecystectomy specimen, diffuse growth pattern was noted in 66 (62.8) cases and polypoidal growth was evident in 39 (37.2%) cases. Mean tumor size was 4.31 cm with a range of 1.0 to 9.0 cm. 83.1% patients had ≥2.5 cm tumor size. Only fundus involvement was noted in 19 (18.0%), only body involvement was noted in 11 (10.4% cases). Fundus and body both were involved in 29 (27.6%) cases. Neck involvement along with body was present in 18 (17.1%). Entire fundus, body and neck involvement was present in 28 (26.6%) cases.
Cases were assigned pathological stage (p stage) as per the AJCC system 8th edition in 105 cases where simple cholecystectomy or radical cholecystectomy specimen were available. In 163 (60.8%) cases with advanced disease (stage 3/4), clinical staging was done along with histological verification of gallbladder carcinoma in small biopsies. In 105 operated patients, T1a, T1b, T2, T3, T4 tumor sizes were noted in 2 (1.9%), 17 (16.2%), 57 (54.3%), 26 (24.8%) and 3 (2.9%) cases respectively. AJCC stage was assigned at 1, 2, 3a, 3b, 4a and 4b in 18 (17.1%), 46 (43.8%), 18 (17.1%), 18 (17.1%), 2 (1.9%) and 3 (2.9%) cases respectively.
Microscopic characteristics of gallbladder carcinoma
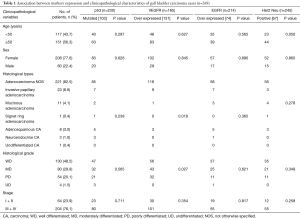
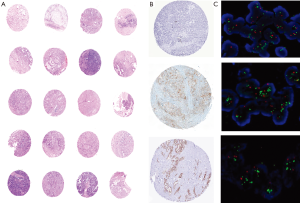
Histological subtyping was done in all 268 cases. Adenocarcinoma not otherwise specified (NOS) synonymous with conventional adenocarcinoma was the most common tumor comprising of 221 (82.5%) cases. An extremely well differentiated adenocarcinoma like morphology with gastric foveolar phenotype was noted in one of the case. Histological grading, based on the extent of glandular formation in the tumor was implemented as per the WHO guidelines (9). Most adenocarcinoma NOS were well to moderately differentiated. Well, moderately and poorly differentiated adenocarcinomas represented 97 (43.9%), 71 (32.1%) and 53 (24.0%) of tumors respectively (Figure 1A,B). Invasive papillary adenocarcinoma (Intracystic papillary neoplasm with an associated invasive carcinoma) was the commonest histological variant, comprising 23 (8.6%) of cases (Figure 1C). Eleven cases of mucinous adenocarcinoma (mucinous cystic neoplasm with an associated invasive carcinoma), showed more than 50% cells with intracellular mucin as well as extracellular mucin. Eight cases showed an adenosquamous morphology with two distinct malignant components, one glandular and the other squamous (Figure 1D). The adenocarcinoma component was mostly moderately differentiated (6 out of 8). One case of signet-ring cell carcinoma was also identified, which show diffuse sheets of tumor cells with intracytoplasmic mucin displacing the nuclei toward the periphery (Figure 1E). By convention, signet-ring cell carcinoma was assigned grade 3. Three cases of immunohistochemically categorized high grade neuroendocrine tumor (NEC) and one case of undifferentiated carcinoma were also included in the study. All three NEC were positive for cytokeratin, synaptophysin, chromogranin with high Ki67 index (Figure 1F). Undifferentiated carcinoma was composed of sheets of round cells with vesicular nuclei and prominent nucleoli along with tumor cell spindling and giant cell change and was categorized as high grade (grade 4). Morphological spectrum was evident in all stages of cancers and there was no significant correlation between various histological subtypes with age group, sex and tumor stage. Histological grading was done in all 268 cases and showed 130 (48.5%) cases of well differentiated adenocarcinoma, 80 (29.9%) moderately differentiated adenocarcinoma. The depth of tumor invasion, adjacent organ involvement, lymphovascular emboli, perineural invasion (PNI) and extent nodal involvement was additionally studied in 65 radical cholecystectomy specimens. Lamina invasion, muscularis propria involvement, perimuscular infiltration and liver infiltration were evident in 2 (3.1%), 11 (16.9%), 25 (38.5%) and 25 (38.5%). Adjacent two extrahepatic organs involvement was seen in 2 (3.1%) cases. Lymphovascular invasion (LVI) and PNI were present in 20 (30.8%) and 7 (10.8%), cases respectively. Lymph node metastasis was evident in 32.3% patients most of whom were N1 nodal stage (30.8%).
Analysis of marker expression and its correlation with clinicopathological parameters
p53, EGFR VEGF and Her2/Neu was expressed in 38.4%, 34.6%, 79.4% and 27.3% percent cases of gall bladder carcinoma. Percentage expression of all four markers in large specimen (radical cholecystectomy and simple cholecystectomy) and biopsies was comparable.
Analysis of p53 overexpression by immunohistochemistry
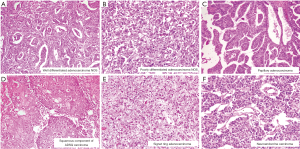
Overall 103 (44.8%) of 230 cases showed mutant protein expression in IHC with positive nuclear expression of 2+ and 3+ intensity in >50% tumor cell nuclei. All neuroendocrine carcinoma (n=3) showed p53 overexpression. Increasing grades of tumor from well differentiated to poorly differentiated carcinoma did not show any correlation with p53 mutation. Intratumoral heterogeneity in expression of p53 was evident in insignificant number of cases. There was no significant difference in p53 expression between primary and metastatic biopsy sites (P=0.634). Further p53 mutational status did not correlate with tumor size (<2.5 vs. ≥2.5 cm) (P=0.160), tumor site (fundus &/body vs. neck) (P=0.658), growth pattern (P=0.866), level of infiltration (P=0.808), LVI (P=0.919), PNI (P=0.141), T (P=0.659), N (P=0.825) and stage (P=0.738) in cholecystectomy specimen (Figure 2A,B,C).
Analysis of EGFR overexpression by immunohistochemistry
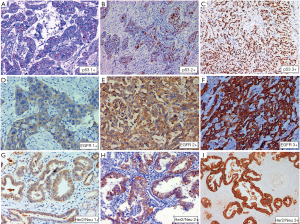
Seventy four (34.6%) of 214 cases tested showed positive EGFR protein expression in IHC defined as positive membranous expression of 3+ intensity in >10% tumor cells. Equivocal cases (2+, >10%) constituted 26.6%. Two of three cases of neuroendocrine carcinoma and one case each of signet ring carcinoma and undifferentiated carcinoma did not show EGFR overexpression. Tumor grade did correlate with EGFR overexpression. Intratumoral heterogeneity was not evident. There was no significant difference in expression between localized disease and metastatic lesions (P=0.948). Further EGFR mutational status did not correlate with tumor size (<2.5 vs. ≥2.5 cm) (P=0.878), tumor site (Fundus, body vs. neck) (P=0.977), growth pattern (polypoidal vs. infiltrating) (P=0.503), level of infiltration (P=0.243), LVI (P=0.475), PNI (P=0.890), T (T1 vs. T2 vs. T3 vs. T4) (P=0.141), N (N0 vs. N1 vs. N2) (P=0.645) and Stage (I vs. II vs. III vs. IV) (P=0.202) in radical cholecystectomy specimen (Figure 2D,E,F).
Analysis of VEGF overexpression by immunohistochemistry
One hundred and thirty one (79.4%) of 165 cases tested showed protein overexpression in IHC with membranous expression of 2+ and 3+ intensity in >30% tumor cells. Complete absence of membranous staining was identified in 10.9% cases only. VEGF overexpression correlated significantly with histological subtypes (P=0.018) and histological grade (P=0.027). Both cases of neuroendocrine tumors and single case of signet ring carcinoma did not show overexpression. All adenosquamous carcinoma (n=3) showed overexpression. VEGF overexpression was highest in moderately differentiated adenocarcinoma (85.1%) and lowest in undifferentiated carcinoma (0%). Intratumoral heterogeneity in expression of VEGF was not evident. There was no significant difference in expression between localized disease and metastatic lesions (P=0.802). Further VEGF overexpression did not correlate with tumor size (<2.5 vs. ≥2.5 cm) (P=0.212), tumor site (Fundus &/body vs. neck) (P=0.277), growth pattern (polypoidal vs. infiltrating) (P=0.945), level of infiltration (P=0.111), LVI (P=0.550), PNI (P=0.483), T (T1 vs. T2 vs. T3 vs. T4) (P=0.262), N (N0 vs. N1 vs. N2) (P=0.232) and stage (I vs. II vs. III vs. IV) (P=0.038) in cholecystectomy specimen.
Analysis of HER2/Neu overexpression by immunohistochemistry
Sixty seven (27.3%) of 245 cases tested showed HER2 protein overexpression in IHC with strong complete or basolateral membranous expression in ≥10% tumor cells. Complete loss of membranous staining was identified in 38.8% cases and 13.1% cases showed 1+ positivity. Twenty percent cases were equivocal (2+, >10%) (Figure 2G,H,I). HER2/Neu overexpression was more common older patients ≥50 years of age (P=0.050). There was no significant difference in HER2 overexpression between histological subtypes and grade groups. However all three cases of neuroendocrine tumors and one case of signet ring carcinoma did not show HER2 overexpression. Intra-tumoral heterogeneity in HER2/Neu expression was evident 13 (20%) cases with adequate tissue, 50% of these had a papillary morphology along with an invasive NOS-like component. The two components showed discrete variation in heterogeneous tumors (Figure 3). There was no significant difference in expression between localized and metastatic lesions (P=0.289). Further HER2 overexpression did not correlate with tumor size (<2.5 vs. ≥2.5 cm) (P=0.802), tumor site (fundus &/body vs. neck) (P=0.585), growth pattern (polypoidal vs. infiltrating) (P=0.845), level of infiltration (P=0.403), LVI (P=0.701), PNI (P=0.129), T (T1 vs. T2 vs. T3 vs. T4) (P=0.241), N (N0 vs. N1 vs. N2) (P=0.487) and Stage (I vs. II vs. III vs. IV) (P=0.265) in cholecystectomy specimen.
Analysis of HER2/Neu overexpression by FISH
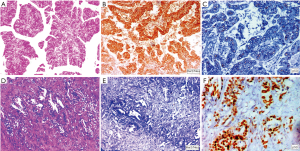
FISH analysis for HER2/Neu overexpression was performed to validate result of HER2/Neu immunohistochemistry. Twenty nine cases examined for FISH included all 16 IHC equivocal cases and 7 positive cases and 6 IHC negative cases. According to Hofmann’s HER2 FISH scoring criteria (10), 8 cases (27.6%) were identified as HER2/Neu gene amplified and the other 21 cases (72.4%) were HER2/Neu gene amplification negative. Two of the 16 equivocal cases were positive in FISH. Six of the seven IHC positive (3+) cases were positive in FISH while the other IHC 3+ positive case showed polysomy. None of the IHC 0 and IHC1+ group tumors demonstrated FISH amplification. Hence a high concordance was detected between positive, negative Her2 FISH and Her2 IHC results (12/13) while in cases with equivocal HER2 IHC score 2+, HER2 FISH was positive in only 12.5% cases (2/16) (Figure 4).
Next generation sequencing
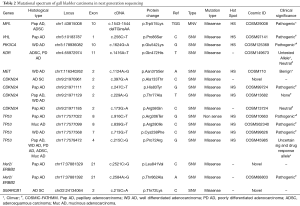
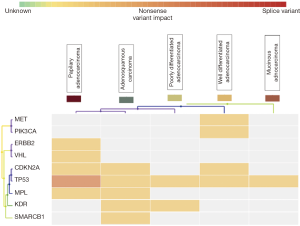
NGS using Ion AmpliSeq cancer hotspot panel V2 revealed multiple non synonymous mutations, most frequent being TP53 and CDKN2A mutations along with mutations in MET, KDR, PIK3CA, VHL, MPL, HER2 and SMARCB1 genes. Gene mutations observed in twelve representative cases of different histological types and grades, including two control samples of cholecystitis have been detailed in Table 2. All mutations were identified in exonic regions of the respective genes and were mostly small nucleotide variations (SNV) with missense mutation. Clinvar identified a known pathogenic role in 8 mutations. Cosmic IDs were assigned on review to all but three mutations which showed up as novel and were identified in SMARCB1, HER2 and CDKN2A genes. Loci and protein change are described in Table 2. Variant impact chart displaying non-synonymous mutations in Figure 5 compares the mutation profile in papillary adenocarcinoma, adenosquamous carcinoma, poorly differentiated adenocarcinoma, well differentiated adenocarcinoma and mucinous adenocarcinoma. Alterations in p53 gene were consistent across all histological subtypes. Mucinous adenocarcinoma showed minimum accumulation of mutations with p53 being a consistent feature. KDR gene mutation was seen in adenosquamous cell carcinoma and adenocarcinomas with poorly differentiated morphology. SMARCB1 mutation was present in adenosquamous cell carcinoma only. Synonymous mutations, possibly non-pathogenic, included FGFR3, PDGFRA, APC, EGFR, MET, CDKN2A, NOTCH1, RET, HRAS, AKT1, TP53 and STK11 alterations.
Full table
Discussion
To the best of our knowledge the current study is the largest retrospective study comprising of 268 cases of gallbladder carcinoma, analyzing HER2 aberrations along with EGFR, VEGF, and p53 alteration in patients. We have further attempted to detail the intratumoral heterogeneity in cases with resections. Expression of markers was correlated with clinicopathological parameters. The study also presents interesting data of genetic alterations in next generation sequencing in gallbladder carcinoma subtypes and grades.
Gallbladder cancer evolution like colonic cancer goes through phases of metaplasia of normal epithelium, or less commonly hyperplasia, then dysplasia or intraepithelial neoplasia, and carcinoma in situ, before becoming an invasive malignancy (13). Mutations of TP53 and mitochondrial DNA are observed at an early stage (14). The appearance of intraepithelial neoplasia is frequently associated with a loss of heterozygosity at loci 3p, 8p and overexpression of HER2 (15,16). Standard statistical analysis of clinicopathological parameters including age, sex, histological subtypes, histological grade as per the WHO criteria and the TNM stage did not show any significant association with EGFR, p53 mutant protein and HER2 expression (Table 1). In view of hypothesis of the evolution of gallbladder carcinoma and since HER2 and p53 mutations have a postulated role in initiation of gall bladder carcinoma, their status would not change significantly with progress of cancer.
A recent study performing whole-exome sequencing of 57 gallbladder cancer tumor-normal pairs showed somatic mutations in EGFR (4%), HER2 (10%), ERBB3 (12%), and ERBB4 (4%) (17). High rates of EGFR alterations with overexpression of HER2 have been reported by Kalekou and Miliaras in about a third of all cases (18). Jeffrey et al. observed HER2 amplification in 16% cases (19). p53 mutations are similar to those in other solid tumors and 40–70% of cases harbor a TP53 mutation (20). However, small case numbers in the some regions have limited these studies. It is also interesting to note that all three neuroendocrine carcinomas in our study also expressed mutant p53 protein.
We have used the Her2/Neu interpretation criteria as for gastric and gastroesophageal cancer (10). The Her2/Neu interpretation criteria in breast cancer and gastric cancer are similar except of the inclusion of basal with basolateral staining as positive (21). All equivocal cases, some negative and positive cases underwent FISH analysis for validation of IHC results. The correlation of positive and negative IHC expression with FISH results was excellent (concordance in 92.3% cases).
Intratumoral heterogeneity in Her2/Neu expression was also investigated in the current study and overall 27.3% cases expressed HER2/Neu. It is interesting to note that 20.8% cases showed a heterogeneous expression. A fair number of heterogeneous cases had a positive expression in papillary exophytic growth while the infiltrative components showed restricted expression of HER2/Neu. Previous reports on HER2/Neu expression patterns in gall bladder cancer are rather limited (22). Yoshida et al. have also observed heterogeneity of HER2/Neu overexpression in 20/39 (51%) of cases with IHC 2+ and 3+ cases, where the staining pattern was heterogeneous (23). Preclinical and clinical data suggest that HER2/Neu could be a therapeutic target. Some cases with HER2/Neu expression have reportedly responded well to anti-HER2/Neu monoclonal antibody (trastuzumab) therapy, with or without taxane (24).
NGS across a variety of histological subtypes including adenocarcinoma well differentiated, adenocarcinoma poorly differentiated, mucinous adenocarcinoma, adenosquamous cell carcinoma showed consistent p53 mutation. Further in the evolution of carcinoma, the lesion accumulates mutations of KRAS and loss of heterozygosity at 9p, 13q and 18q (25,26). These genetic aberrations are believed to drive the lesion to develop into an invasive carcinoma (27). Churi et al. in a study of mutation profiling in cholangiocarcinoma (CCA) observed that there were significant differences in frequency of the genetic alterations between intrahepatic and extrahepatic CCA (28). Commonly occurring genes mutated were TP53 (35%), KRAS (24%), ARID1A (20%), IDH1 (18%), MCL1 (16%) and PBRM1 (11%) in intrahepatic CCA. In extrahepatic CCA were TP53 (45%), KRAS (40%), ERBB2 (25%), SMAD4 (25%), FBXW7 (15%) and CDKN2A (15%) (28). In our analysis using NGS for 50 hotspots, we have observed mutations in TP53 and CDKN2A genes along with mutations in MET, KDR, PIK3CA, VHL, MPL, HER2 and SMARCB1 which are synchronous with the extrahepatic type of CCA. RAS-RAF-MEK-MAPK signaling axis plays a crucial driver of growth of invasive carcinoma (29).
Vascular endothelial growth factor A (VEGF-A) is a potent proangiogenic agent involved in the carcinogenesis of many human tumors. The role of VEGF expression in predicting post treatment clinical outcomes of gallbladder carcinoma by estimating its expression in relation to known prognostic indicators has also been analysed in the current study. Overall VEGF overexpression correlated significantly with histological subtypes (P=0.018) and histological grade (P=0.027). Other parameters including stage, size, nodal status did not correlate significantly with VEGF overexpression. Sun XN in a series of 84 patients reported a high expression of VEGF-A at 53.6% (30). VEGF-A expression in their study had a positive correlation with metastatic disease and histological differentiation. The nodal status and VEGF-A expression were independent prognostic factors of overall survival in their patients. Letelier et al. reported a high expression of VEGF-A in 81% (183/224) in cases of gall bladder cancers and 5.1% (2/39) of chronic cholecystitis (P<0.0001) (31). Liu et al. further estimated serum levels of VEGF-C and VEGF D in cases and controls. Significant high levels of VEGF C and D were present in serum of cases vs. controls. These levels were associated with lymph node metastasis, distant metastasis, and stage (32). It appears that increasing VEGF expression is related to progression of gallbladder carcinoma.
The current study is limited by the fact that NGS was done in representative cases (two of each histological subtype) and hence statistical significance of mutations in terms of differences between histological subtypes, grade and stage of lesion could not be elicited. It was however interesting to note that p53 mutations were present across the spectrum of histological types including papillary adenocarcinoma, adenocarcinoma NOS well differentiated, poorly differentiated adenocarcinoma, adenosquamous carcinoma and mucinous adenocarcinoma. Further pathological staging could be done in 105 of cholecystectomy. Other cases were in clinical stage 3 or 4 with unresectable disease, where diagnosis, IHC and molecular studies were done in small biopsy samples obtained in tru-cut or open wedge biopsies. In some cholecystectomy specimen nodal requirement of the retrieval of a minimum number of six nodes could not be fulfilled (8).
The strength of the current study is that it addresses a large cohort of cases with gallbladder cancer in which expression of four markers has been studied. We have used stringent criteria for cutoffs for positive expression of markers. Co-expressions and heterogeneity issues have been addressed. For HER2/Neu, equivocal IHC and concordance in negative and positive cases has been validated using FISH. Good correlation suggests that an HER2/Neu scoring system using gastric criteria can be implemented in gall bladder carcinoma. Positive marker expression in high fraction of cases has identified a significant subgroup of GBC cases, in which targeted therapy may increase survival of patients.
Conclusions
Targetable markers like HER2/Neu, VEGF and EGFR are expressed in high proportion of gallbladder carcinoma. RAS pathway molecules like HER2/Neu and EGFR showed significant expression suggesting that interactions take place among different members of the ErbB family during tumor development. We also found appreciable intratumoral heterogeneity in HER2/Neu expression. We identified a significant subgroup of gallbladder cancer cases, in which targeted therapy may increase survival of patients. Clinical trials with anti-HER2/Neu, anti-EGFR and anti-VEGR therapy may be considered especially in countries with a high incidence of gallbladder malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by Institutional Ethical committee of King George’s Medical University, Lucknow India Reference code: 70th ECM 11-A/P6 and Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow Reference code: IEC 2/14 and written informed consent was obtained for all patients before performing study-related procedures. Waiver of consent was obtained for archival material.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 2016;7:797-803. [Crossref] [PubMed]
- Ménard S, Tagliabue E, Campiglio M, et al. Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol 2000;182:150-62. [Crossref] [PubMed]
- Mitri Z, Constantine T, O'Regan R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother Res Pract 2012;2012:743193. [Crossref] [PubMed]
- Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 2007;5:203-20. [Crossref] [PubMed]
- Gonzalez-Angulo AM, Hortobágyi GN, Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist 2006;11:857-67. [Crossref] [PubMed]
- Sohal DP, Shrotriya S, Abazeed M, et al. Molecular characteristics of biliary tract cancer. Crit Rev Oncol Hematol 2016;107:111-8. [Crossref] [PubMed]
- Amin MB, Edge SB, Green FL, et al. AJCC Cancer Staging Manual. 8th Edition, NewYork: Springer, 2017:79-94.
- Bosman FT, Carneiro F, Hruban RH, et al. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Digestive System. (Fourth Edition). Lyon: IARC Press, 2010;253-6.
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [Crossref] [PubMed]
- Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF and HER2 expression in cholangiocarcinoma. British Journal of Cancer 2008;98:418-25. [Crossref] [PubMed]
- Pais-Costa SR, Farah JF, Artigiani-Neto R, et al. Evaluation of P53, E-cadherin, Cox-2, and EGFR protein immunoexpression on prognostic of resected gallbladder carcinoma. Arq Bras Cir Dig 2014;27:126-32. [Crossref] [PubMed]
- Marcano-Bonilla L, Mohamed EA, Mounajjed T, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016;5:61. [Crossref] [PubMed]
- Wistuba II, Albores-Saavedra J. Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobiliary Pancreat Surg 1999;6:237-44. [Crossref] [PubMed]
- Wistuba II, Maitra A, Carrasco R, et al. High resolution chromosome 3p, 8p, 9q and 22q allelotyping analysis in the pathogenesis of gallbladder carcinoma. Br J Cancer 2002;87:432-40. [Crossref] [PubMed]
- Chaube A, Tewari M, Garbyal RS, et al. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patient’s. BMC Cancer 2006;6:126. [Crossref] [PubMed]
- Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 2014;46:872-6. [Crossref] [PubMed]
- Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c-erbB2 in gallbladder carcinoma. J Gastroenterol Hepatol 2004;19:812-8. [Crossref] [PubMed]
- Jeffrey SR, Kai W, Daniel VT, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and genomic alterations. J Clin Oncol 2015;33:abstr 231.
- Javle M, Rashid A, Churi C, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol 2014;45:701-8. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Kaseb A. A phase II study trastuzumab (NSC 688097) in Her2/Neu positive cancer of the gallbladder or biliary tract (NCI 7756). Available online: http://clinicaltrials.gov/show/NCT00478140, accessed 20 Dec 2014.
- Yoshida H, Shimada K, Kosuge T, et al. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Arch 2016;468:431-9. [Crossref] [PubMed]
- Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. J Clin Oncol 2012;30:e271-3. [Crossref] [PubMed]
- Barreto SG, Dutt A, Chaudhary A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann Oncol 2014;25:1086-97. [Crossref] [PubMed]
- Chang HJ, Kim SW, Kim YT, et al. Loss of heterozygosity in dysplasia and carcinoma of the gallbladder. Mod Pathol 1999;12:763-69. [PubMed]
- Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol 2016;22:1335-47. [Crossref] [PubMed]
- Churi CR, Shroff R, Wang Y, et al. Mutation Profiling in Cholangiocarcinomaa, Prognostic and Therapeutic Implications. PLoS One 2014;9:e115383. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84. [Crossref] [PubMed]
- Sun XN, Cao WG, Wang X, et al. Prognostic impact of vascular endothelial growth factor-A expression in resected gallbladder carcinoma. Tumour Biol 2011;32:1183-90. [Crossref] [PubMed]
- Letelier P, Garcia P, Leal P, et al. Immunohistochemical expression of vascular endothelial growth factor A in advanced gallbladder carcinoma. Appl Immunohistochem Mol Morphol 2014;22:530-6. [Crossref] [PubMed]
- Liu MC, Jiang L, Hong HJ, et al. Serum vascular endothelial growth factors C and D as forecast tools for patients with gallbladder carcinoma. Tumour Biol 2015;36:6305-12. [Crossref] [PubMed]


