Can we downstage locally advanced pancreatic cancer to resectable? A phase I/II study of induction oxaliplatin and 5-FU chemoradiation
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States (1). The 5-year survival rate of patients with newly diagnosed disease remains about 8% (1). The high incidence of metastatic disease at diagnosis and the relative chemo-resistance of this tumor contribute to this poor survival rate. Long term survival is only possible with curative resection. However, only 15% to 20% of patients present with disease confined to the pancreas at the time of diagnosis and as such deemed resectable (2): approximately 40% have distant metastases, and another 30% to 40% have tumors that extend outside the pancreas, in absence of distant metastases. Two terms are currently used by the National Comprehensive Cancer Network (NCCN) guidelines in order to identify this last category of patients: “borderline resectable” and “locally advanced unresectable disease”. The difference between these groups relates to the degree of invasion of the regional vasculature (portal vein-superior mesenteric vein confluence, celiac axis and superior mesenteric artery) by tumor, and to the possibility of performing an adequate vascular resection and reconstruction during the operation (3,4). Upfront surgery in this category of patients is either not technically feasible or likely to lead to microscopically positive margins of resection, which does not seem to confer a survival benefit compared with no resection (5). Therefore, neoadjuvant therapy is the appropriate treatment strategy in this setting, with the purpose of controlling local disease and converting to resectable (4,6,7).
Around the end of the last century and the beginning of the new one, some data was published in favor of the use of induction chemotherapy and radiotherapy followed by surgery in the treatment of pancreatic cancer (8-13). Results, however, were not unanimous, with some studies still reporting no survival benefit and a significant higher toxicity for the combined treatment modality (14). Randomized trials, therefore, were being started to assess the new strategy, as the 2000-01 FFCD/SFRO study (15), evaluating the role of radiation together with 5-FU and cisplatin versus gemcitabine alone, or the intergroup study lead by ECOG, comparing radiation therapy plus gemcitabine with gemcitabine alone (16).
In 1997, NYU had undertaken a phase I/II evaluation of a novel combination of Gemcitabine/Cisplatin combined with radiation in patients with locally advanced unresectable disease. The tested regimen was well tolerated and yielded good tumor control, but was limited in its ability to render locally advanced disease resectable (17).
At that time, oxaliplatin had been identified as a promising new agent, with greater activity compared to cisplatin and a demonstrated in vitro cytotoxic effect against pancreatic cancer cell lines (18,19). Furthermore, a synergistic effect of oxaliplatin in combination with 5FU had already been demonstrated, preclinically (20), in metastatic colorectal cancer patients (21), and even in patients with metastatic pancreatic cancer (22). Such a combination, moreover, had been tested in association with radiotherapy in patients with recurrent or locally advanced rectal cancer, and the regimen was well tolerated (23).
Based on promising pre-clinical data and the need for more effective therapy in combination with radiation for loco-regionally advanced pancreatic cancer, we designed a phase I/II study to test the safety and efficacy of combined weekly infusional 5-FU and oxaliplatin with concurrent radiotherapy.
Methods
Eligibility and evaluation
Patients with locoregionally advanced pancreatic carcinoma were enrolled at New York University Medical Center and Bellevue Hospital Center. The protocol (NYU 03-64) was approved by the NYU School of Medicine Institutional Review Board (FWA 00004952), which oversees both participating institutions, and written informed consent was obtained for all patients before performing study-related procedures.
Eligibility criteria included pathologic diagnosis of pancreatic adenocarcinoma (PC), locoregionally advanced, non-metastatic disease on computed tomography (CT) imaging, Eastern Cooperative Oncology Group (ECOG) performance status ≤1, and adequate bone marrow, liver and renal function. When the study was initiated, the current NCCN guidelines to define resectability status had not been established yet. Borderline resectable and unresectable diseases were retrospectively defined according to the M.D. Anderson criteria for resectability, which were subsequently incorporated into the guidelines of National Comprehensive Cancer Network (NCCN) (4).
Exclusion criteria included histology other than adenocarcinoma, metastatic disease, prior chemotherapy and/or radiotherapy, active uncontrolled infection, inadequate respiratory, renal, cardiac, hepatic or hematologic organ function, and pregnancy.
Pre-study evaluation and staging included a complete history and physical examination, chest radiograph, blood analysis (complete blood cell count, basic metabolic profile, coagulation profile, and liver function tests), carcinoembryonic antigen and cancer antigen 19-9 levels, and abdomen/pelvis CT or magnetic resonance imaging. Staging laparoscopy was performed in selected cases.
Throughout and after various phases of treatment, patients were followed regularly, through regular reevaluations of ECOG performance status, physical examinations including body weight, and laboratory values. Serial CT or magnetic resonance imaging was used to reevaluate disease stage after administration of chemoradiation (CH-RT) treatment. When feasible, post-treatment follow-up was pursued every 3 months until patient death.
Study regimen and design
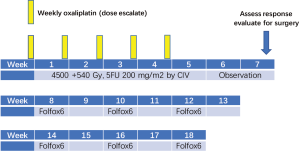
Radiation consisted of 4,500 cGy in 25 fractions (180 cGy/fx daily) over 5 weeks, followed by a comedown to the tumor and margins for an additional 540 cGy in 3 fractions, for a total dose of 5,040 cGy in 28 fractions over 5 and a half weeks.
Radiation was combined with 5FU 200 mg/m2 daily by continuous infusion for 5 weeks and weekly oxaliplatin for 5 weeks in dose escalation cohorts as following: level I =30 mg/m2; level II =40 mg/m2; level III =50 mg/m2; level IV =60 mg/m2. Following the phase I portion of the trial, a phase II trial at the recommended dose continued.
Two weeks following completion of CRT, patients were re-staged with CT scan. Those considered resectable underwent surgery; those who remained unresectable for cure (stable disease, SD) but did not progress (PD) received a modified FOLFOX6 (Oxaliplatin 85 mg/m2 administered at day 1 as a 2-hour IV infusion concurrently with Leucovorin 350 mg administered as a 2-hour IV infusion, followed by 5-FU 400 mg/m2 as an IV bolus, followed by 5-FU 2,400 mg/m2 as a 46-hour infusion) every 2 weeks for 6 cycles (Figure 1).
Throughout treatment, patients were evaluated at least weekly by history, physical examination, and laboratory values to monitor for toxicity.
Toxicity was graded according to the NCI Common Toxicity Criteria (NCI CTC), Version 2.0 (24). Neurosensory toxicity was graded according to the Neurologic Toxicity Scale for Oxaliplatin (25).
Treatment toxicity including gastrointestinal symptoms, fever, fatigue, neutropenia, thrombocytopenia, anemia, and high liver function tests was monitored. Appropriate chemotherapy and/or radiation dose modification was performed accordingly; the dose was held for absolute neutrophil count <500 cells/µL, platelet count <25,000/µL, grade 3–4 non-hematologic toxicity (except neurologic toxicity and grade 3 diarrhea). Treatment was resumed when absolute neutrophil count >1,000/µL, platelets >50,000/µL, resolution of non-hematologic toxicity to grade 2 or less. If toxicity required a dosing delay of more than three weeks from the last planned Oxaliplatin dose, study treatment was discontinued.
Dose-limiting toxicity was defined as: prolonged grade 4 neutropenia or complicated grade 3–4 neutropenia (fever >38.5 °C or sepsis); grade 4 thrombocytopenia or symptomatic grade 3–4 thrombocytopenia (hemorrhage); any other grade 4 toxicity of clinical relevance that is not reduced to grade 1 within 2 days of appropriate therapy.
Primary endpoint for the phase I portion of the study was to determine the safety and the MTD of the combined CIV5FU, oxaliplatin and radiation. Secondary endpoints were rates of R0 resectability and overall survival (OS), calculated from time since first treatment. Primary endpoint for the phase II portion was resectability rate, with secondary outcomes including overall survival and toxicity.
Resectability rate included the proportion of patients who successfully underwent complete surgical resection with microscopically negative margins as a function of all patients treated with CH-RT therapy. Survival was measured from the date of start of treatment to the time of death from any cause.
Statistical analyses
A standard 3+3 cohort dose-escalation design was utilized for the phase I portion of the trial. The recommended phase II dose was defined as the highest dose tested in which none or one patient experienced dose-limiting toxicity attributable to the study drug. At least six patients were treated at the recommended phase II dose.
Overall survival time was illustrated using Kaplan-Meier curves. Descriptive statistics were provided according to the nature of variables. All analyses were performed with SPSS statistical software, version 13.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
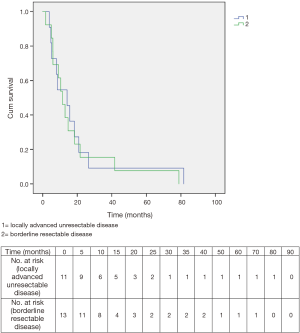
Between June 2004 and December 2009, 15 men and 9 women were enrolled in the study. Demographics and tumor characteristics are reported in Table 1. The mean age was 64.5 (range, 46–76) years. A total of 16 cancers arose in the head/neck of the pancreas. The median tumor size at presentation as measured radiographically by computed tomography was 3.75 (range, 1.50–7.90) cm. Thirteen tumors were classified retrospectively as “borderline resectable” and eleven as “unresectable”.

Full table
Maximum tolerated dose and toxicity
CH-RT
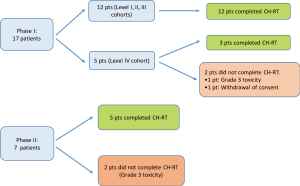
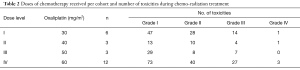
Seventeen patients were enrolled in the phase I portion of the study. They received radiation therapy combined with daily 5-FU and weekly oxaliplatin in 4 dose cohorts. Six patients were included in cohort 1 (oxaliplatin 30 mg/m2), three patients in cohort 2 (oxaliplatin 40 mg/m2), three patients in cohort 3 (oxaliplatin 50 mg/m2) and five patients in cohort 4 (oxaliplatin 60 mg/m2).
All of the 12 patients in the first three cohorts completed 5 weeks of treatment without need for dose reduction. Among the 5 patients in level 4 cohort, three patients completed the treatment, one patient developed grade 3 toxicity (gastritis and dehydration) that mandated interruption of treatment, and one patient withdrew consent for research. The highest dose (60 mg/m2) of oxaliplatin, thus, was well tolerated and it was therefore carried forward in the phase II portion of the study.
Seven patients were enrolled in the phase II portion of the study and they all received Oxaliplatin at a dose of 60 mg/m2. One patient developed grade 3 toxicities (mucositis, lymphopenia, fatigue) which did not allow for completion of the 5 weeks. Another patient had to interrupt the treatment because of grade 3 lymphopenia. Overall, 5 patients out of 7 in the phase II portion completed CH-RT.
A schematic of patients’ enrollment and number of patients who completed CH-RT treatment is shown in Figure 2.
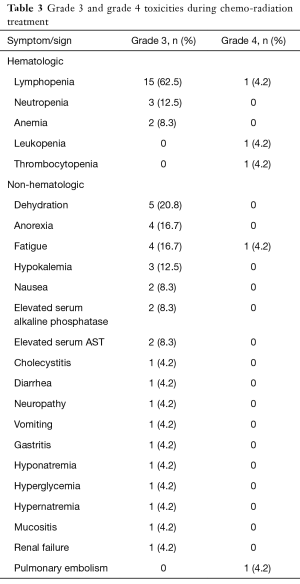
Overall number of toxicities within each cohort and specific grade 3/4 toxicities are summarized in Table 2 and Table 3. Overall, grade 4 toxicities related to initial CH-RT were observed during phase I (n=2, pulmonary embolism and lymphopenia) and phase II (n=3, fatigue, leukopenia and thrombocytopenia).
Full table
Full table
Folfox6
Fourteen patients started additional chemotherapy with Folfox6: eleven from phase I (of the initial 17) and three from phase II (of the initial 7). As will be described in detail next section, these were patients who had stable, still unresectable disease after CH-RT, either at imaging or at exploratory laparotomy, plus one patient who had regression of disease and received Folfox6 after radical resection.
The eleven phase I patients included four out of six patients from cohort 1, two of three patients from cohort 2, all three patients from cohort 3 and two of five patients from cohort 4. Nine of these 11 patients were able to complete all 6 cycles. Treatment was stopped in 2 patients, one for clinical progression of disease and one for development of toxicity.
Of the three phase II patients that started Folfox6, none completed the treatment. One patient withdrew consent after 3 cycles. One patient experienced toxicity that precluded continuation of treatment after the second cycle. Finally, the last patient experienced a significant regression during Folfox6 after the second cycle, appeared to have become resectable, and was brought to the operating room for a pancreaticoduodenectomy.
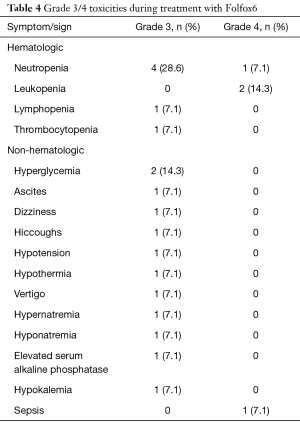
Grade 3/4 toxicities during treatment with Folfox6 are reported in Table 4.
Full table
Overall, grade 4 toxicities related to additional treatment with Folfox6 were observed twice during phase I (one each leukopenia and sepsis) and twice in phase II (one each leukopenia and neutropenia).
Overall patient response
The overall response of patients to treatment as they progressed through the protocol is outlined in Figure 3.
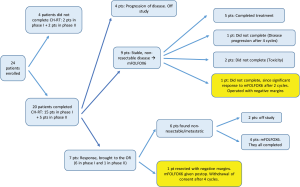
Twenty-four patients began CH-RT treatment. Of those, 20 patients (15 from phase I and 5 from phase II) completed it. Reasons for stopping CH-RT were grade 3 toxicity (n=3) and withdrawal of consent (n=1).
Of the twenty patients who completed the oxaliplatin-based CH-RT, at re-staging after CH-RT, 4 patients had progressive disease.
Seven patients (29.2%) were noted to have a response following CH-RT, and were offered exploratory laparotomy and potential resection. Of these seven patients, 5 initially had borderline resectable disease, and 2 had locally advanced unresectable disease. Of these patients, however, 6 were found with non-resectable disease at the time of surgery: 2 with carcinomatosis and 4 with stable, but non-resectable disease. Only 1 patient was found to be resectable at the time of surgery, and received radical resection. This patient had originally presented with a cT2N0M0, 3.8-cm mass of the uncinate process, and was deemed borderline resectable on retrospective analysis, based on current NCCN guidelines (4). Postoperative pathology revealed a 2 cm mass, T1N1M0, resected with negative microscopic margins.
Nine patients had stable, non-resectable disease following CH-RT and received additional chemotherapy with Folfox6. Five completed all 6 cycles of Folfox6; one patient had disease progression after 4 cycles, and two patients stopped their treatment due to toxicity after 2 and 4 cycles, respectively; finally, one patient demonstrated a dramatic tumor response after the second Folfox6 treatment cycle and underwent curative intent resection at another institution. She had originally presented with a cT4N0M0 mass of the head of pancreas, retrospectively deemed borderline resectable. Outside postoperative pathology revealed a T3N0M0 adenocarcinoma, resected with negative microscopic margins.
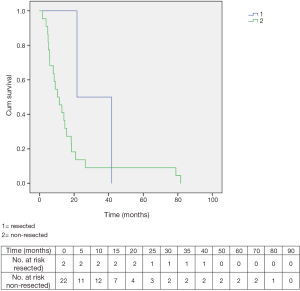
Overall, thus, two patients (8.3%) received curative resection.
Follow-up and survival
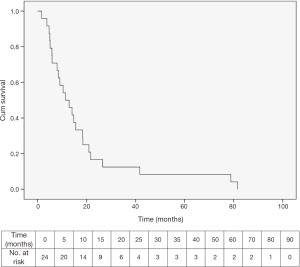
There is complete follow up on all patients and all 24 patients have died of disease.
The median survival for the entire study group was 11.4 (range, 1.7–81.6) months (Figure 4).
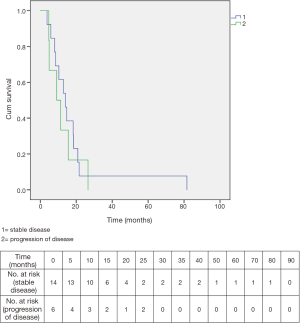
Among the 20 patients who completed CH-RT, median overall survival was 12.9 months. Fourteen of these twenty had stable disease after CH-RT: their median survival was 14.1 months. Six of the 20 who completed CH-RT showed immediate progression or were explored and found to have progression on the basis of carcinomatosis and their median survival was only 9.1 months. The two survival curves are shown in Figure 5.
Overall survival for patients initially diagnosed with borderline resectable disease was 11.4 months, while it was 14.1 months for patients originally deemed to have locally advanced unresectable disease. The difference was not significant (Figure 6).
The two resected patients had a survival of 41.7 and 21.6 months, respectively. Overall survival in non-resected patients was 10.4 months (Figure 7).
Discussion
The present report demonstrates that combined modality treatment for NCCN-designated borderline resectable and locally advanced unresectable pancreatic cancer with Oxaliplatin, 5-FU and radiation was reasonably well tolerated in our phase I/II study. The majority of patients in the study (91.7%), however, remained unresectable. Of note, two patients who were resected had negative margins on postoperative pathology. Survival data with the tested regimen were comparable to other studies for locally advanced pancreas cancer, with a better outcome, as expected, for those patients who had stable disease or were resected versus those who progressed on study.
At present, surgical resection is the main curative modality for the adenocarcinoma of the pancreas. However, in the setting of NCCN-defined borderline resectable or locally advanced unresectable disease, resection for cure (defined as both gross and microscopically negative margins) is generally not possible. In addition, an R1 surgical resection does not confer a survival benefit compared with no resection (5). The use of neoadjuvant therapy, therefore, is reasonable in for this patient population in order to control local disease, prevent development of metastases and to possibly downstage to a resectable status, thus maximizing the potential for an R0 resection (4).
At the time when our study was initiated, several protocols had already demonstrated the potential benefit for induction chemotherapy and radiation therapy followed by surgery in the treatment of pancreatic cancer (8-12,26,27).
In 1997, NYU undertook a phase I/II evaluation of a novel combination of gemcitabine/cisplatin before and combined with radiation in patients with locally advanced unresectable pancreatic cancer. The regimen was well tolerated and allowed for good tumor control, although was limited in converting locally advanced disease to resectable (17).
The present trial was conceived with a similar design, but with different agents. Oxaliplatin was chosen for its superior pre-clinical efficacy in pancreatic cancer, its radiosensitizing properties, and its synergistic effect with 5-FU (18-23,28).
In terms of our primary endpoint of toxicity for this regimen, oxaliplatin fared quite well. One of the issues regarding the use of oxaliplatin is its potential neurotoxicity, which is considered its dose-limiting factor. The most common acute side effect is a transient peripheral neurotoxicity characterized by paresthesia and dysesthesia in hands, feet and the perioral area, triggered and/or enhanced by contact with cold. These symptoms, though, are dose-dependent, becoming observable usually at doses of 90 mg/m2, and, interestingly, disappear within 12 weeks after stopping treatment in 50% of patients and in the majority after 30 weeks (29). The maximum dose used in our study, however, was 60 mg/m2: only one patient developed grade 3 neuropathy (4.2%).
At doses higher than 45 mg/m2, oxaliplatin induces nausea and vomiting with rapid onset in the great majority of patients, but this is usually controlled by the standard anti-emetic measures used for all platinum derivatives. We only had two cases of grade 3 nausea (8.3%), and one single case of grade 3 vomiting (4.2%) during induction CH-RT. Furthermore, as expected, no significant renal toxicity was associated with the use of oxaliplatin. The regimen was overall well tolerated, and the most frequent single hematologic toxicity was lymphopenia, with 15 grade 3 episodes (62.5%) and 1 grade 4 episode (4.2%) (overall grade 3–4: 66.7%). Among non-hematologic toxicities, dehydration, with 5 grade 3 episodes (20.8%), was most common. Other grade 3–4 toxicities are summarized in Table 3.
Overall, 20 patients out of 24 completed CH-RT treatment (83.3%).
Other studies assessing combination regimens in advanced pancreas cancer with oxaliplatin have reported similar or higher rates of toxicity.
The phase III GERCOR/GISCAD Intergroup trial randomized 326 patients with locally advanced or metastatic unresectable pancreatic cancer to either gemcitabine alone or gemcitabine (1 g/m2 every 2 weeks) + oxaliplatin (100 mg/m2 every 2 weeks). It reported an overall good tolerance of the combination therapy, although with a higher incidence of grade 3 and 4 thrombocytopenia, vomiting, neurosensory symptoms, nausea and neutropenia in the Gem/Ox arm (30).
A prospective, phase II clinical trial by Sahora et al. evaluated gemcitabine (900 mg/m2) and oxaliplatin (60 mg/m2) weekly for patients with locally advanced, non-metastatic pancreatic cancer: the most common toxicities observed were neutropenia/leukopenia (25%) and peripheral neuropathy (18%); diarrhea was described in 4% of patients and vomiting in 14% (31).
Other combination regimens have been reported in advanced diseases that were not oxaliplatin based. Grade 3/4 toxicities were again similar or higher (32,33).
Finally, the use of gemcitabine plus nab-paclitaxel is in the current NCCN guidelines as a first-line therapy option for locally advanced pancreatic cancer (4), although no data have been published to date in this group of patients: all available evidence is derived from patients with metastatic disease. The phase III multinational randomized trial in metastatic disease reported grade 3 or 4 toxicity in the gemcitabine/nab-paclitaxel arm of neutropenia (38%), thrombocytopenia (13%), febrile neutropenia (3%), fatigue (17%), diarrhea (6%), and neuropathy (17%) (34). These data show an overall higher rate of adverse events than our regimen, although data regarding survival are not comparable because of the different type of studied population. A modified regimen of gemcitabine + nab-paclitaxel has recently been proposed for metastatic cancer, and a preliminary report presented at 2015 ASCO Gastrointestinal Cancer Symposium showed similar survival rates with a better grade 3–4 toxicity profile: neutropenia 10%, fatigue 6%, neuropathy 2%, thrombocytopenia 4%, diarrhea 0% (35). However, even with a better toxicity profile, the use of gemcitabine/nab-paclitaxel is costly, currently the highest among the most common regimens (36).
Our survival results are comparable with those of other regimens for treatment of locally advanced pancreatic cancer.
Among those studies which evaluated the use of Gemcitabine in mixed populations of patients with both locally advanced and metastatic disease (30,32,37), the GERCOR/GISCAD trial showed combined therapy to be superior to monotherapy only in terms of response rate (26.8% vs. 17.3%), progression-free survival (5.8 vs. 3.7 months), but not for median overall survival, which was 9.0 and 7.1 months, respectively (30).
A phase II study by Ishii et al., instead, focusing exclusively on locally advanced pancreatic carcinoma, reported a median overall survival of 15.0 months for 50 patients treated with gemcitabine alone (38).
Thirteen patients (39%) had a curative resection after neoadjuvant therapy with gemcitabine and oxaliplatin in the study by Sahora et al.: median overall survival of patients undergoing resection was 22 months, as opposed to 12 months for those without resection (31).
In the last few years, combination chemotherapy with FOLFIRINOX has become a standard regimen in metastatic disease based on randomized data (39), for patients with a good performance status, a favorable comorbidity profile, and a support system to permit aggressive medical therapy. Although it appears that objective response rates in the primary tumor are at least as good as they are in metastatic disease, few data and no randomized studies are today available for locally advanced unresectable disease (40-46).
However, a systematic review and meta-analysis of studies for locally advanced pancreatic cancer patients treated with FOLFIRINOX has recently been published in which 490 patients across ten studies were included: the median overall survival was of 24.2 (range, 10.0–32.7) months; the proportion of patients who underwent surgical resection ranged from 0% to 43%, with a pooled proportion of 25.9%. The pooled proportion of patients who had R0 resection of those who underwent resection was 78.4% (47).
With respect to borderline resectable disease, no randomized data exist. The use of neoadjuvant therapy in patients with borderline resectable disease has been evaluated only in small single institution series and small phase II trials. The largest single institution series included 160 patients; of these, 125 (78%) completed preoperative therapy and restaging, and 66 (41%) underwent pancreatectomy. Sixty-two patients (94%) had a margin-negative postoperative pathology; at a median follow-up of 27 months, the median survival durations for the unresectable and resectable cohorts were 13 and 40 months, respectively (48). Interestingly, a 2011 meta-analysis of phase II trials testing a variety of neoadjuvant strategies concluded that approximately one third of tumors initially considered marginal for resection were able to be ultimately resected after neoadjuvant treatment and that the median survival in this group was 22.3 (range, 18–26) months (49). In general, current recommendations from NCCN in the setting of borderline resectable disease are for an initial attempt at neoadjuvant therapy, followed by restaging and surgical exploration in the absence of metastatic disease, rather than upfront surgery (4). This is particularly appropriate, when considering that borderline resectable tumors are usually more likely to have a curative resection as opposed to a truly defined locally advanced unresectable disease (17). In fact, both patients who underwent curative resection in our study were originally classified as borderline resectable.
Overall, despite its limited size, our phase I/II trial has demonstrated that the addition of 5-FU and oxaliplatin to radiation therapy in the neoadjuvant setting for patients with locally advanced unresectable and borderline resectable pancreatic tumor is feasible and well tolerated. However, the greater majority of patients remained unresectable after treatment, and survival rates were comparable to those shown by other reports about different therapy combinations. Thus, more novel approaches will have to be tested in order to truly improve outcomes in patients with locally advanced unresectable and borderline resectable disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol (NYU 03-64) was approved by the NYU School of Medicine Institutional Review Board (FWA 00004952), which oversees both participating institutions, and written informed consent was obtained for all patients before performing study-related procedures.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma (Version 1.2016). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol 2006;13:1201-8. [Crossref] [PubMed]
- Seufferlein T, Bachet JB, Van Cutsem E, et al. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii33-40. [Crossref] [PubMed]
- Balaban EP, Mangu PB, Khorana AA, et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2654-68. [Crossref] [PubMed]
- Haller DG. Chemotherapy for advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2003;56:16-23. [Crossref] [PubMed]
- Douglass HO. Treatment of Locally Unresectable Carcinoma of the Pancreas - Comparison of Combined-Modality Therapy (Chemotherapy Plus Radiotherapy) to Chemotherapy Alone. J Natl Cancer Inst 1988;80:751-5. [Crossref] [PubMed]
- Heinemann V. Gemcitabine-based combination treatment of pancreatic cancer. Semin Oncol 2002;29:25-35. [Crossref] [PubMed]
- Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys 1994;30:161-7. [Crossref] [PubMed]
- Hoffman JP, Weese JL, Solin LJ, et al. Preoperative chemoradiation for patients with resectable pancreatic adenocarcinoma. An Eastern Cooperative Oncology Group (ECOG) phase II study. Proc Am Soc Clin Oncol 1995;14:201.
- Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 1988;80:751-5. [Crossref] [PubMed]
- Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373-8. [Crossref] [PubMed]
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9. [Crossref] [PubMed]
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Marti JL, Hochster HS, Hiotis SP, et al. Phase I/II trial of induction chemotherapy followed by concurrent chemoradiotherapy and surgery for locoregionally advanced pancreatic cancer. Ann Surg Oncol 2008;15:3521-31. [Crossref] [PubMed]
- Scheeff ED, Briggs JM, Howell SB. Molecular modeling of the intrastrand guanine-guanine DNA adducts produced by cisplatin and oxaliplatin. Mol Pharmacol 1999;56:633-43. [Crossref] [PubMed]
- Kornmann M, Fakler H, Butzer U, et al. Oxaliplatin exerts potent in vitro cytotoxicity in colorectal and pancreatic cancer cell lines and liver metastases. Anticancer Research 2000;20:3259-64. [PubMed]
- Raymond E, Buquet-Fagot C, Djelloul S, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs 1997;8:876-85. [Crossref] [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [Crossref] [PubMed]
- Ducreux M, Mitry E, Ould-Kaci M, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol 2004;15:467-73. [Crossref] [PubMed]
- Aschele C, Friso ML, Pucciarelli S, et al. A phase I-II study of weekly oxaliplatin (OXA), 5-fluorouracil (FU) continuous infusion (CI) and preoperative radiotherapy (RT) in locally advanced rectal cancer (LARC). Proc Am Soc Clin Oncol 2002:abstr 527.
- Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:13-47. [Crossref] [PubMed]
- Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol 2003;30:5-13. [Crossref] [PubMed]
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of Locally Unresectable Pancreatic-Carcinoma - a Randomized Comparison of High-Dose (6000 Rads) Radiation Alone, Moderate Dose Radiation (4000 Rads + 5-Fluorouracil), and High-Dose Radiation +5-Fluorouracil. Cancer 1981;48:1705-10. [Crossref] [PubMed]
- Rich T, Harris J, Abrams R, et al. Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12. Am J Clin Oncol 2004;27:51-6. [Crossref] [PubMed]
- Woynarowski JM, Chapman WG, Napier C, et al. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol 1998;54:770-7. [Crossref] [PubMed]
- Garufi C, Levi F, Giunta S, et al. Chronomodulated 5-day infusion of floxuridine and L-folinic acid in patients with advanced malignancies: a feasibility and tolerability study. J Infus Chemother 1995;5:134-7. [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [Crossref] [PubMed]
- Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149:311-20. [Crossref] [PubMed]
- Lima CMR, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [Crossref] [PubMed]
- Reni M, Cereda S, Balzano G, et al. Outcome of upfront combination chemotherapy followed by chemoradiation for locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 2009;64:1253-9. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Krishna K, Blazer MA, Wei L, et al. Modified gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (MPC): A single-institution experience. J Clin Oncol 2015;33:366. [Crossref]
- Goldstein DA, Krishna K, Flowers CR, et al. Cost description of chemotherapy regimens for the treatment of metastatic pancreas cancer. Med Oncol 2016;33:48. [Crossref] [PubMed]
- Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430-8. [Crossref] [PubMed]
- Ishii H, Furuse J, Boku N, et al. Phase II Study of Gemcitabine Chemotherapy Alone for Locally Advanced Pancreatic Carcinoma: JCOG0506. Jpn J Clin Oncol 2010;40:573-9. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236-41. [Crossref] [PubMed]
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199. [Crossref] [PubMed]
- Marthey L, Sa-Cunha A, Blanc JF, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol 2015;22:295-301. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153-9. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Sadot E, Doussot A, O'Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol 2015;22:3512-21. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [Crossref] [PubMed]
- Assifi MM, Lu X, Eibl G, et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 2011;150:466-73. [Crossref] [PubMed]