Management of liver metastases from gastrointestinal stromal tumors: where do we stand?
Introduction
Gastrointestinal stromal tumors (GISTs) constitute the most frequently encountered mesenchymal tumors of the gastrointestinal tract, accounting for 1–3% of all its malignancies (1,2). Although the most common primary site of GISTs is the stomach and proximal small intestine, they can be found in anywhere along the GI tract (1). It is estimated that 25% of patients with newly diagnosed GISTs present with synchronous metastatic disease, usually in the liver and peritoneum (3). Other locations include the lungs and bones (4).
The management of metastatic GIST disease has evolved dramatically during the last decade as a result of the introduction of tyrosine kinase inhibitors (TKIs) (5). Response to traditionally used chemotherapeutic agents for metastatic GIST lesions until the 00’s was poor. On the contrary, the initial response to imatinib has been demonstrated to be as high as 80% (6). Survival rates before TKI did not exceed 16 months. Post introduction of imatinib, patients enjoy a remarkable overall survival (OS) of approximately 5 years (7). Imatinib has become the base therapy for metastatic GISTs. Indications for surgical resection as recommended by the National Comprehensive Cancer Network (NCCN) guidelines include limited disease, progression refractory to TKI, and locally advanced or previously unresectable tumors that manifest favorable response to neo adjuvant therapy with TKI (8).
The timing, sequence, and effectiveness of imatinib administration and surgical resection have been the subject of debate. In the absence of randomized controlled trials, recommendations and current strategies are based on small volume studies. The aim of our review was to evaluate currently available information on long-term results following resection of liver metastases (LM) from GISTs.
Methods
Study design
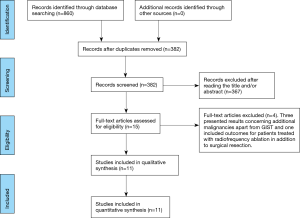
Our systematic review followed PRISMA guidelines (9). All appropriate observational studies (prospective and retrospective) addressing postoperative outcomes in patients with liver resection from metastatic GIST were included in the systematic review (Figure 1). Case reports, reviews and animal studies were excluded from analysis and tabulation. Nikolaos Machairas, Anastasia Prodromidou and Ioannis D. Kostakis independently searched the literature, excluded overlapping data, and tabulated the selected indices in structured forms. Consensus of all authors resolved potential discordances in methodology, selection of articles, and statistical analysis.
Search strategy and data collection
We searched for articles published up to October 2016 using Medline [1966–2016], Scopus [2004–2016], and Google Scholar [2004–2016] databases with the references included in the full text articles retrieved. The following key words were used for the search: “liver metastasis”, “gastrointestinal stromal tumors”, “metastasectomy”, “liver resection”, “metastatic GIST”, “liver surgery”. A minimum number of search keywords were utilized in an attempt to assess an eligible number that could be easily searched while simultaneously minimizing the potential loss of articles. Those articles that fulfilled or were deemed to fulfill the inclusion criteria were retrieved. The PRISMA flow diagram schematically presents the stages of article selection (Figure 1).
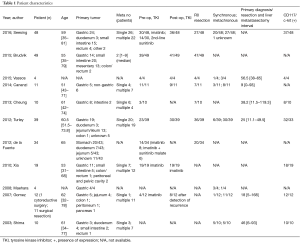
Data on patient characteristics included age, primary tumor location, single or multiple LM, preoperative and/or postoperative TKI administration, R0 resection, synchronous and metachronous metastases, interval from primary tumor diagnosis to detection of liver metastasis, and CD117 c-kit positivity.
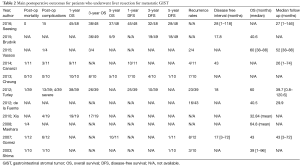
Survival rates were evaluated as follows: 1-, 3-, 5-year OS, 1-, 3-, 5-year disease free survival, as well as disease free survival (DFS) and OS intervals. Mortality and morbidity following surgery as well as recurrence rates were appraised. Finally, we examined potential predictors of survival after liver resection.
Definitions
Postoperative mortality was defined as the overall number of recorded deaths within 30 days following surgery. The terms DFS and recurrence-free survival (RFS) were considered as identical.
Results
A high heterogeneity in the selection of populations, study characteristics, and discrepancies in the interpretation of statistical analyses of individual studies precluded a meta-analysis of the results. A meticulous systematic review was therefore conducted.
Included and excluded studies
Four studies were excluded from our analysis (10-13). Three of them reported results on additional malignancies and did not fulfill our inclusion criteria (10,11,13). The forth study in addition to including LM from various sarcomas also encompassed radiofrequency ablation as a therapeutic strategy (12).
Eleven studies comprising 240 patients diagnosed with LM from GISTs originating at various locations were included in the present systematic review (14-24). The analyzed indices were structured in three tables as follows: patient characteristics (Table 1), main outcomes (Table 2), and potential predictors of postoperative outcomes (Table 3).
Full table
Full table

Full table
Characteristics of included studies and potential bias
Seesing et al. retrospectively described 48 patients who underwent LM resection from GIST (14). Patients lost to follow up were excluded. TKI therapy was administered to thirty patients preoperatively (200–800 mg daily of imatinib, or sunitinib as a second-line treatment) and to 36 postoperatively. Twenty-seven underwent R0 resections.
Cananzi et al. included 11 patients with metastatic GIST to the liver who underwent hepatic resections following neoadjuvant TKI therapy (imatinib) in a retrospective study (22). Patients were preoperatively divided into two groups: the ‘progression group’ (where cytoreductive surgery was performed) and the ‘response to TKI group’ (where surgery with curative intent was undertaken). Two individuals did not receive therapy with TKI. R0 resections were reported in seven cases. Survival rates as well as the association between R0 resection and postoperative deaths were recorded.
Brudvik et al. reported results from 146 patients with liver metastasis after GIST or sarcoma (15). In the present review we selectively evaluated 49 patients with GIST LM included in the above mentioned study. A total of 39 received preoperative TKI and 41 postoperative TKI. Apart from reporting OS, relapse-free survival outcomes, and recurrence rates, a subgroup analysis on adjuvant imatinib and relapse free survival was also performed. Univariate analysis revealed prognostic factors of survival.
Vassos et al. encompassed 12 patients diagnosed with hepatic metastases from GIST (16). Patients diagnosed with liver failure were excluded. Liver resection was performed in four patients, all of which received pre-and postoperative TKI therapy. R0 resection was achieved in all cases. Survival outcomes and recurrence rates were separately recorded for patients undergo surgery.
Cheung et al. evaluated postoperative survival outcomes in 10 patients with GIST LM in a retrospective study (17). Extrahepatic metastases, technically unresectable lesions, liver cirrhosis and/or poor functional hepatic reserve with potential residual liver volume <30% represented exclusion criteria. Three of the 10 patients received preoperative TKI treatment with imatinib; 70% underwent R0 resection.
Turley et al. included 39 patients who underwent GIST liver metastasectomy in a retrospective study (18). Patients with cirrhosis and abnormal liver function tests were excluded. TKI was administered as neoadjuvant therapy prior to surgery in 23 patients; 19 of them were treated with imatinib for a median interval of 18 months; 4 received sunitinib for a median of 8.5 months. Postoperatively, imatinib was administered in 27 patients and sunitinib in 6 (due to imatinib resistance). Uni- and multi-variate analyses were conducted to identify predictive factors of survival and to clarify the association between TKI timing and resection.
de la Fuente et al. included 59 patients in a retrospective comparative study (19). A total of 43 patients with hepatic metastases were compared to 16 with synchronous hepatic metastases and sarcomatosis; 34 patients underwent hepatectomy for single hepatic metastases; 14 of them received preoperative TKI therapy (8 imatinib and imatinib and/or sunitinib). The effectiveness of this study in evaluating the potential benefits of surgery was limited by the lack of survival outcomes of resected patients.
Xia et al. reported 39 patients in a randomized trial (23); 19 of them (the operation group) received a combination of surgical resection with preoperative (6 months) and postoperative (2–4 weeks) oral imatinib. The non-operation group included the remaining 20 who received imatinib as the only treatment. A subgroup analysis based on the extent of response to TKI therapy was conducted to evaluate survival among the two groups.
Gomez et al. included 12 patients aged 32–78 years in a retrospective single center trial (20). Preoperatively four received imatinib for a median period of 13.5 months. Two of them developed recurrences and were treated with TKI (imatinib) postoperatively. Imatinib was also administered to all other patients with recurrences (n=8). Clinico-pathological features of patients with disease free survival greater than and under 2 years were evaluated.
Shima et al. described 10 patients treated with hepatectomy for GIST LM (21). No significant differences were reported for median survival based on timing, number, and size of hepatic metastases. Mortality and survival rates were listed.
Finally, Maehara et al. presented a series of four patients who underwent hepatic resection after gastric GIST (24). In addition to the limited number of enrolled patients, data on survival outcomes were limited to mortality and median survival.
Postoperative mortality and complications
Results addressing postoperative mortality were analyzed in seven studies (14,17,18,20,22,24). Two recorded no postoperative deaths after metastasectomy (14,17). In the remaining five studies, one patient was not alive in the immediate interval following surgery (18,20,22,24). Eight studies addressed postoperative morbidity for both surgical and non-surgical complications (14,16-18,20-23). Seesing et al. detected 15 patients with postoperative complications, three of which required reoperations for bleeding and bile leakage (14). Gomez et al. had six patients with postoperative complications. Four had intra-abdominal collections and two wound infections (20). Vassos et al. and Shima et al. noted only one patient each with postoperative complications, a pleural effusion and hearth failure, respectively. Cheung et al. described no complications (16,17,21). Xia et al. had complications in four patients in the operation group, none of which required reoperations (23). Finally Turley et al. and Cananzi et al. had complications in 13 (33%) and 3 (27.3%) patients, respectively. Among them, four and two patients respectively suffered from severe complications (Clavien-Classification III–V) (18,22).
OS
Eight studies reported OS rates (14-18,20,22,23). Concerning 1-year OS, Cheung et al. and Xia et al. reported 100% 1-year OS (17,23). Seesing et al. and Turley et al. consistently presented similar OS rates (93% and 96.7%, respectively) (14,18). Cananzi et al. reported a somewhat inferior rate of 80.8% (22). Six studies reported results for 3-year OS (14-18,23). In the studies of Seesing et al. and Xia et al., the 3-year OS was 80% and 89.5% respectively (14,23). A reduced 3-year OS was found by Brudvnik et al., Vassos et al., Turley et al. and Cheung et al. (72.7%, 75%, 75% and 67.9%, respectively) (15-18). With respect to 5-year OS, Gomez et al. reported a 91% rate. The corresponding rates for Seesing et al., Cheung et al. and Brudvik et al. were 76%, 50%, and 55.3%, (14,15,17,20). A median OS interval of 41.8 months was calculated based on data retrieved from six studies (15,16,18-21).
DFS and recurrence rates
Five studies monitored DFS among patients who underwent liver metastasectomy from GIST. Similarly, in the study of Seesing et al. the interval among liver metastasectomy and recurrence or progression of disease was defined as progression-free survival (PFS). The 1-year DFS was 87.5% in the study by Cananzi et al. (22). Rates of 63.4% and 70% were observed by Cheung et al. and Turley et al., respectively (17,18). Three studies reported 3-year DFS of 39%, 42% and 26.1% (15,17,18). Five-year DFS was 14%, 10% and 11% according to Cheung et al., Gomez et al. and Shima et al., respectively (17,20,21). Gomez et al. found no significant clinico-pathological features associated with disease-free intervals greater than or under 2 years (20). Seesing et al. reported 1-, 3- and 5-year PFS of 93%, 67%, and 59%, respectively and a median PFS of 89 [1–121] months. A median DFS of 17.9 [17–43] months following resection was calculated based on data retrieved from four studies.
Seven studies included recurrence rates (16-22). A total of 63 out of 129 patients presented with recurrent disease after liver metastasectomy. Vassos et al. and Gomez et al. mentioned that recurrences occurred in two and eight patients respectively and were treated with surgery and/or RFA (16,20). Seven of ten patients in the study of Cheung et al. developed tumor recurrence (17). In the same study, none of the risk factors examined as predictive of recurrence (such as age, mitotic activity and tumor characteristics) reached significance. Shima et al. and Cananzi et al. recorded similar recurrences (three and four patients, respectively) in the peritoneum and/or the liver (21,22). Finally, 23 and 16 patients were diagnosed with recurrent disease after liver metastasectomy by Turley et al. and de la Fuente et al. (18,19).
Predictors of postoperative outcome
Table 3 shows outcome predictors as demonstrated by the included studies. R0 resection was reported as the most discriminating variable associated with a positive outcome in patients with liver metastasis from GIST who underwent any kind of liver resection. Particularly, in the study of Seesing et al. multivariate analysis revealed a significant improvement of OS in patients who underwent R0 resection (P=0.02) (14). By univariate analysis, TKI therapy administered preoperatively was significantly related to improve OS. Nevertheless, multivariate analysis found no significant difference in OS among patients who received TKI therapy (either preoperatively and/or postoperatively) and those who were treated with surgery alone. Cananzi et al. reported a significantly improved survival for R0 vs. R1/R2 resections (P=0.001) (22). In the study of Brudvik et al. a combination of resection and TKI therapy with imatinib was found to be significantly superior to surgery alone. Univariate analysis found only age greater than 55 years to be a significant predictor of poor outcome (P=0.013) (15). Multivariate analysis by Turley et al. defined postoperative TKI and extrahepatic disease as significant confounders of OS (P=0.006 and P=0.012, respectively) (18). Finally, in the study of Xia et al. a significantly improved survival was found in patients who underwent resection and TKI therapy when compared to those who did not have surgery (P=0.04) (23).
Discussion
The treatment strategy for GISTs has undergone a major evolution during the last 15 years as a result of TKIs. The introduction of these targeted agents has remarkably improved survival rates even in the setting of metastatic disease. Surgical resection of LMs from GISTs had historically been considered as a palliative measure (11). In the past, patients with LM from sarcomas treated with surgery alone had median survivals of 37–39 months (10,12). Recent studies however, have highlighted the beneficial effect of surgical resection in the treatment of metastatic GISTs in combination with TKI administration, reporting 5-year survival rates as high as 91% (14,20).
Poor initial response and acquired resistance associated with chronic use constitute important limitations of imatinib (25). Notably, acquired resistance to imatinib has been reported in almost half of patients after 18 months (6). A recent multicentric study demonstrated that patients who underwent surgery for metastatic GIST disease after prolonged preoperative administration of TKIs had worse outcomes than those who received short neoadjuvant TKI treatments (26). Although the authors remarked that such results could be explained by a selection bias of patients with less desirable responses, they reached the conclusion that surgical resection confers maximum benefit only when undertaken within a short “window of opportunity” (26).
The evaluation of predictive factors has also been problematic due to the small volume of patients and the differences in therapeutic approaches used during the years. Reported predictive factors include surgical resection, R0 resection, age <55 years, clinical response to TKIs, pre- and post-operative administration of TKIs (14,15,18,22,23). The most recent study by Seesing et al. reported an R0 resection rate of 56% and its significant prognostic relevance by both uni- and multivariate analyses (14). Brudvik et al. as well as Turley et al. reported R0 resection rates of 96% and 92% respectively, but failed to evaluate the actual influence of R0 resections as a predictor of OS (15,18). Although the study by de la Fuente reported an R0 resection rate of 59% that could have potentially provided valuable information on its prognostic value, the authors did not proceed with a specific analysis (19). Of note, a recently published large series of patients with metastatic disease from large-volume European centers also highlighted the importance of R0 resections. R0/R1 vs. R2 surgeries had a median OS of 8.7 vs. 5.3 years respectively (P=0.001), resulting in a survival benefit of approximately 3 years (25). The results of these studies highlight the importance of a step-wise approach with neo-adjuvant TKIs followed by complete surgical resections. The characteristics of patients who could potentially benefit from a debulking approach based on either poor/no response or from progression of disease still remain to be determined.
A previous review by Zalinski et al. proposed a combination of TKI treatment and hepatic resection, highlighting the importance of neoadjuvant TKI therapy in the selection of patients suitable for resection (27). Similar observations were reported in the review by Ye et al. who described an increased possibility of R0 resections with the introduction of TKI therapy (28). Finally, another recent review reported that patient selection in combination with timing of resection based on the outcomes of TKI treatment constitutes the most crucial factors in the management of GIST patients with liver metastasis (29).
The great heterogeneity in the studies considered, combined with the small number of patient described, prevented us from reaching further conclusions. Surgical resection combined with TKIs in the adjuvant and neo-adjuvant setting seems to constitute the most efficient treatment. Further well-designed prospective randomized controlled studies are needed to determine which patients with metastatic disease from GISTs will benefit from this multidisciplinary approach as well as to define the optimal sequence and duration of treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol 2000;15:1293-301. [PubMed]
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am 2013;42:399-415. [Crossref] [PubMed]
- Lamba G, Gupta R, Lee B, et al. Current management and prognostic features for gastrointestinal stromal tumor (GIST). Exp Hematol Oncol 2012;1:14. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11:4182-90. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2014. J Natl Compr Canc Netw 2014;12:473-83. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg 2001;234:540-7; discussion 547-8. [Crossref] [PubMed]
- Nunobe S, Sano T, Shimada K, et al. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol 2005;35:338-41. [Crossref] [PubMed]
- Pawlik TM, Vauthey JN, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:537-43; discussion 543-4. [Crossref] [PubMed]
- Rehders A, Peiper M, Stoecklein NH, et al. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg 2009;33:111-7. [Crossref] [PubMed]
- Seesing MF, Tielen R, van Hillegersberg R, et al. Resection of liver metastases in patients with gastrointestinal stromal tumors in the imatinib era: A nationwide retrospective study. Eur J Surg Oncol 2016;42:1407-13. [Crossref] [PubMed]
- Brudvik KW, Patel SH, Roland CL, et al. Survival After Resection of Gastrointestinal Stromal Tumor and Sarcoma Liver Metastases in 146 Patients. J Gastrointest Surg 2015;19:1476-83. [Crossref] [PubMed]
- Vassos N, Agaimy A, Hohenberger W, et al. Management of liver metastases of gastrointestinal stromal tumors (GIST). Ann Hepatol 2015;14:531-9. [PubMed]
- Cheung TT, Chok KS, Chan AC, et al. Analysis of long-term survival after hepatectomy for isolated liver metastasis of gastrointestinal stromal tumour. ANZ J Surg 2014;84:827-31. [Crossref] [PubMed]
- Turley RS, Peng PD, Reddy SK, et al. Hepatic resection for metastatic gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Cancer 2012;118:3571-8. [Crossref] [PubMed]
- de la Fuente SG, Deneve JL, Parsons CM, et al. A comparison between patients with gastrointestinal stromal tumours diagnosed with isolated liver metastases and liver metastases plus sarcomatosis. HPB (Oxford) 2013;15:655-60. [Crossref] [PubMed]
- Gomez D, Al-Mukthar A, Menon KV, et al. Aggressive surgical resection for the management of hepatic metastases from gastrointestinal stromal tumours: a single centre experience. HPB (Oxford) 2007;9:64-70. [Crossref] [PubMed]
- Shima Y, Horimi T, Ishikawa T, et al. Aggressive surgery for liver metastases from gastrointestinal stromal tumors. J Hepatobiliary Pancreat Surg 2003;10:77-80. [PubMed]
- Cananzi FC, Belgaumkar A, Lorenzi B, et al. Liver surgery in the multidisciplinary management of gastrointestinal stromal tumour. ANZ J Surg 2014;84:937-42. [Crossref] [PubMed]
- Xia L, Zhang MM, Ji L, et al. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today 2010;40:936-42. [Crossref] [PubMed]
- Maehara N, Chijiiwa K, Eto T, et al. Surgical treatment for gastric GIST with special reference to liver metastases. Hepatogastroenterology 2008;55:512-6. [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40:412-9. [Crossref] [PubMed]
- Zalinski S, Palavecino M, Abdalla EK. Hepatic resection for gastrointestinal stromal tumor liver metastases. Hematol Oncol Clin North Am 2009;23:115-27. ix. [Crossref] [PubMed]
- Ye YJ, Gao ZD, Poston GJ, et al. Diagnosis and multi-disciplinary management of hepatic metastases from gastrointestinal stromal tumour (GIST). Eur J Surg Oncol 2009;35:787-92. [Crossref] [PubMed]
- Mastoraki A, Toliaki E, Chrisovergi E, et al. Metastatic Liver Disease Associated with Gastrointestinal Stromal Tumors: Controversies in Diagnostic and Therapeutic Approach. J Gastrointest Cancer 2015;46:237-42. [Crossref] [PubMed]