A semi-automated assessment of sarcopenia using psoas area and density predicts outcomes after pancreaticoduodenectomy for pancreatic malignancy
Introduction
Despite improvements in the perioperative mortality after pancreaticoduodenectomy (PD), long term survival has not improved significantly in the past 20 years. Complete surgical resection is the only potentially curative therapy for pancreatic adenocarcinoma. Despite modern surgical techniques, PD is associated with complication rates in excess of 50% with long term survival of 20% for resectable disease (1). Although age alone is not a contraindication to PD, patients who are elderly and potentially more frail, tolerate such complications poorly which may delay or preclude their ability to receive adjuvant chemotherapy (2-5). Thus, an objective preoperative risk assessment tool in conjunction with risk factors included in the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) surgical risk calculator (http://riskcalculator.facs.org) would be valuable to help determine which patients are at increased risk for complications and poor outcomes after PD. This would allow surgeons to more accurately discuss the risks and benefits of PD with the patient and family and help guide clinical management.
Frailty has been associated with an increased risk of adverse events following major abdominal surgery due to an impaired ability to recover from physiologic injury (6-12). Sarcopenia, defined as significant loss of skeletal muscle volume and strength has also been associated with poor surgical outcomes (13-17). We previously found that a prospective clinical geriatric assessment (GA) of frailty in patients undergoing PD predicts major complications, longer hospital stays, discharge to a rehabilitation facility, and hospital readmissions (18). In an updated cohort of PD patients, we further demonstrated that sarcopenia significantly correlated with NSQIP serious complications, higher grade of Clavien-Dindo complications, unplanned intensive care unit (ICU) admissions, and discharge to a skilled nursing facility (SNF) (19).
Growing evidence suggests that sarcopenia measurements based on computed tomography (CT) scans can be utilized to predict sarcopenia-related adverse events in patients undergoing PD. In a separate prospective contemporary cohort, we analyzed the ability of preoperative CT-based sarcopenia metrics—measured both manually and with a semi-automated technique—to predict post-PD adverse outcomes among patients undergoing PD for pancreatic cancer.
Methods
Study population
Between 2007 and 2014, patients aged 18 years or older undergoing evaluation for pancreatic surgery at NorthShore University Health System were entered into a prospective database. All patients who ultimately underwent PD with available preoperative abdominal CT scans were included in the current analysis. The Institutional Review Board at NorthShore University Health System approved the study protocol.
Clinical and operative characteristics
Preoperative clinical variables included age, gender, body mass index (BMI), serum albumin, and American Society of Anesthesiologists (ASA) preoperative risk score. A modified Charlson comorbidity score was determined for each patient based on history of cardiac disease, chronic obstructive pulmonary disease, stroke, diabetes mellitus, and active smoking status. Operative factors including type of PD, pancreatic gland texture and duct size, estimated blood loss (EBL), and operative time were recorded.
Preoperative imaging analysis
Sarcopenia was assessed both manually by a trained single observer and using SliceOMatic (Tomovision) by a trained radiologist while blinded to surgical outcomes. The psoas muscle was assessed at the L3 lumbar vertebra at the level of the transverse processes on preoperative CT. The psoas muscle cross-sectional area and attenuation were measured as estimates of psoas muscle volume and density, respectively (20). Psoas muscle volume was estimated with available CT scans and normalized to the patient’s height using the total psoas area index (TPAI) as previously described (19).
Psoas muscle density was estimated for those patients with pre-contrast phase CT scans using the average attenuation in Hounsfield units (HU) which has been shown to correlate with fatty infiltration (21).
Semi-automated measurement of TPAI was performed using thresholds of –30 to 110 HU to calculate psoas muscle area by excluding areas of gross fatty infiltration. Semi-automated analysis of the DICOM images was performed using SliceOmatic (Tomovision) software as described by Peng et al. (17,22). HU was also automatically calculated for these regions.
Postoperative outcome measures
Postoperative recorded outcomes included complications, major complications (Clavien-Dindo III and above), unplanned ICU admission, length of initial hospital stay (LOS), discharge to a SNF, 90-day readmission, and 30-day mortality. Surgical site infections (SSI) were categorized as superficial, deep (including fascial dehiscence), and organ/space (including pancreatic fistula and abscess). Pancreatic fistula was defined according to the International Study Group on Pancreatic Fistula (ISGPF) classification (23). Disposition upon discharge was determined by the attending surgeon incorporating recommendations from physical therapy assessments, patient and family preferences, and social work evaluations. Disease recurrence was based on documented radiographic evidence during cancer surveillance.
Statistical analyses
Descriptive statistics were computed for the clinical variables and expressed as percentages or means with standard deviations, as appropriate. Correlation analyses were performed between sarcopenia measures and outcomes of interest. A series of univariate and multivariate logistic and linear regression models, chosen based on outcomes, were used to assess the predictive value of sarcopenia measures while controlling for hypothesized important clinical covariates from the literature and the NSQIP surgical risk calculator (24). Given the sample size, we limited the models to six potential independent predictor variables (25) including age, BMI (26-28), ASA score (29), serum albumin (30), a modified Charlson comorbidity score, and a measure of sarcopenia (TPAI or HU) in the multivariate analysis. Logistic regression was performed for the dichotomous outcomes of complications, discharge to SNF, and 90-day readmission. Linear regression was used for LOS, except where noted. A predictor with a β coefficient or odds ratio (OR) with a P<0.05 was considered statistically significant. All variables with a P<0.20 were included in the multivariable analysis. A Bland-Altman plot was used to assess the degree of agreement between the manual and semi-automated techniques. All statistical analyses were performed using the SAS 9.4 software (SAS Inc., Cary, NC, USA).
Results
A total of 223 patients underwent a PD during this time period with 183 preoperative CT scans available for review. Of those patients, 116 patients had a diagnosis of a pancreatic malignancy and were included in this study. For the HU assessment, 66 precontrast phase CT scans were available for analysis. Table 1 summarizes the baseline characteristics of the study population which shows that 53% of patients were male with a mean age of 65.5 years, ranging from 39 to 83 years old. The mean BMI was 25.8 kg/m2; 30% were overweight and 18% were obese. Thirty percent of patients had a modified Charlson comorbidity score of 2 or higher. Sixty-four percent of patients had an ASA score of 3 or 4. Nineteen percent of patients had a serum albumin of less than 3.0 g/dL. The mean TPAI was 2.75 cm2/m2 (2.80 cm2/m2 by manual technique) and the mean HU of the psoas at the L3 level was 41.1 (48.3 by manual technique).

Full table
The majority of patients underwent a pylorus-preserving PD (57%), with 17% requiring a vascular reconstruction (Table 2). Of the cases with documented gland texture and duct size, 13% were soft and 28% had small ducts (<3.0 mm). The average length of surgery was 6 hours. The outcomes listed in Table 3 show that 73% of patients had complications, with 17% being major Clavien III or higher complications. The majority of the complications were due to superficial SSI, deep space SSI, and organ/space SSI (36%, 9%, and 13% respectively). Pancreatic fistula rate was 7% with half of those also having an organ/space SSI. There was one unexpected ICU admission, and the average hospital LOS was 12.1 days. There was one postoperative mortality. Upon discharge, about a quarter of the patients (23%) went to a SNF. The hospital readmission rate was 15% within 90 days. With a median long term follow up of 33 months, half of the patients had disease recurrence. Fifteen percent of patients received neoadjuvant chemotherapy or chemoradiation and 91% received adjuvant chemotherapy.
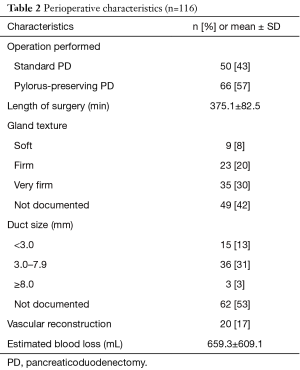
Full table
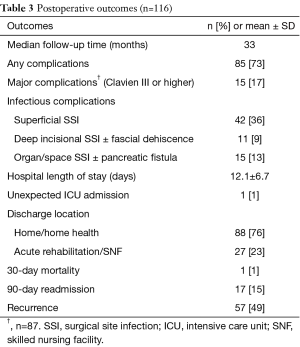
Full table
On univariate analysis, a higher TPAI was associated with an increased risk of organ/space SSI or pancreatic fistula (OR 3.12, P=0.019) (Table 4). Semi-automated assessments of sarcopenia based on lower TPAI (OR 0.34, P=0.009) and HU (OR 0.84, P=0.002) were strongly predictive of discharge to SNF. Sarcopenia, however, was not associated with increased rate of major complications. Using the same set of variables, older age correlated with LOS (β=0.14, P=0.13), discharge to SNF (OR 1.13, P=0.0002), and 90-day readmission (OR 1.06, P=0.04). Variables associated with superficial SSI included low serum albumin (OR 0.27, P<0.001) and firm gland texture (OR 3.86, P=0.04). Low serum albumin was the only variable associated with deep SSI including fascial dehiscence (OR 0.32, P=0.03). Age, comorbidities, albumin, TPAI and HU were all significantly associated with discharge to SNF (Table 4). The only variables associated with increased LOS were albumin (β=−2.42, P=0.02) and age (β=0.14, P=0.01).
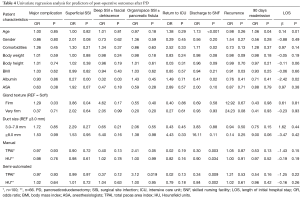
Full table
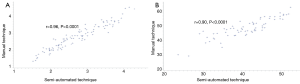
On multivariate analysis (Table 5), the semi-automated measurements of TPAI and HU remained as independent predictors of organ/space SSI including pancreatic fistula (OR 4.23, P=0.014) and discharge to SNF (OR 0.79, P=0.019) respectively. Gland texture and duct size did not appear to affect the risk of any SSI in this cohort. There was also no association between any of the variables and disease recurrence. The semi-automated method strongly correlated strongly with the manual measurements for both the TPAI (r=0.96, P<0.0001) and the HU (r=0.90, P<0.0001) (Figure 1).
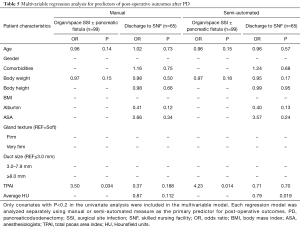
Full table
Discussion
We demonstrated that the semi-automated technique correlates with the manual measurements of TPAI and HU yet is a more reliable predictor of post-PD outcomes. This is most likely due to a more objective measure of muscle size and attenuation as well as the exclusion of gross fatty infiltration achieved by our pre-defined HU parameters. However, the role of fully automated techniques, including comprehensive morphomic analysis, that assess core muscle volume, visceral and subcutaneous adiposity, bone density, and vascular calcifications, might further improve risk-stratification based on preoperative CT (31). Morphomic analysis has also been linked to outcomes after major abdominal operations as well as long-term outcomes after neoadjuvant therapy for resectable pancreatic cancer (32-35). Amini et al. showed that sarcopenia defined as the lowest sex-specific quartile of total psoas volume was a better than the total psoas area at predicting post-operative morbidity and long term survival in patients undergoing pancreatectomy for pancreatic adenocarcinoma (35). However, in an attempt to balance predictive value and ease of clinical implementation, the semi-automated assessments of TPAI and HU appear to be useful independent predictors of post-PD outcomes.
In patients undergoing PD for a pancreatic malignancy, radiographic sarcopenia calculated by semi-automated measurements of HU, was an independent predictor of discharge to a SNF accounting for other preoperative risk factors such as age, BMI, malnutrition, and comorbidities. Furthermore, sarcopenia based on TPAI was associated with discharge to a SNF and a decreased risk of post-operative organ/space SSI including pancreatic fistula. The latter association was unexpected, but may be due to the high proportion of firm glands and large ducts in this cohort. It may also be a function of decreased pancreatic exocrine function (36) or decreased total body water (37) in elderly sarcopenia patients. Of note, sarcopenia was not predictive of post-operative major complications, length of stay, or readmissions. Based on these results, it appears that both poor psoas muscle density and psoas muscle volume are statistically significant predictors of short-term negative outcomes after PD. Although sarcopenia did not appear to predict disease recurrence in these patients, long term cancer outcomes will need to be further analyzed in this cohort.
These results are congruent with the postoperative outcomes reported by Sur et al. from a separate cohort of patients undergoing PD which showed that HU correlated with NSQIP serious complications, Clavien-Dindo complication grade, return to ICU, and discharge to SNF (19). We found that radiographic assessments of sarcopenia were independent predictors of outcomes when adjusting for many of the risk factors included in the NSQIP risk calculator and may be an adjunctive tool to better risk stratify potentially frail patients undergoing PD.
Objective preoperative assessments of frailty are becoming more important as more elderly patients are being considered for surgical resection for pancreatic malignancies. Surgeons have the responsibility of conveying accurate risk assessments and facilitating informed decision-making for patients who are candidates for PD. In addition to the NSQIP surgical risk calculator, semi-automated measures of sarcopenia is an objective, readily available adjunctive tool in the assessment of frailty for patients undergoing major abdominal surgery (13-17).
Although a clinical geriatric frailty assessment was not conducted in this study, the ability to predict poorer outcomes in sarcopenia patients undergoing major abdominal surgery such as PD is a powerful tool that can be used to guide the investigation of actionable interventions to improve outcomes such as prehabilitation programs for sarcopenia patients. In patients receiving neoadjuvant chemotherapy for pancreatic cancer, early identification of those at highest risk of sarcopenia-related complications can facilitate a strengthening and nutritional regimen to empower patients and optimize outcomes. With the apparent decreased risk of organ/space SSI and pancreatic fistula in sarcopenia patients, it may further help guide surgeons with more selective surgical drain placement in patients undergoing PD.
Our study was limited by the modest sample size secondary to limiting HU assessments to patients with a precontrast phase CT scan. This did not enable us to perform gender-specific analyses. However, this was accounted for by normalizing the psoas area to the patient’s height using the TPAI. At this time, no standard method to measure sarcopenia, nor a standard value that defines radiographic sarcopenia has been established. However, using this technique, we have been able to reproduce predictive measures of sarcopenia in two separate cohorts of patients undergoing PD. Compared to our previous study, this cohort focused on patients with pancreatic malignancy who may be at an overall higher risk for poor outcomes due to cancer-related cachexia.
In summary, our study corroborates prior studies which show the use of a semi-automated CT sarcopenia metric that estimates psoas muscle size and density, can add important independent predictive value to clinical risk factors for outcomes after PD for pancreatic cancer. Further prospective studies should be performed to determine whether preoperative sarcopenia assessments of core muscle volume and density are accurate surrogates for surgical frailty and poor outcomes, and whether risk-reducing interventions can minimize adverse post-operative complications in this patient population.
Acknowledgements
This study was funded by the friends of Jack Karp and Barbara Turf and the Rolfe Pancreatic Cancer Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board at NorthShore University Health System (IRB00000549) and written informed consent was obtained from all patients.
References
- Mohammed S, Fisher WE. Quality metrics in pancreatic surgery. Surg Clin North Am 2013;93:693-709. [Crossref] [PubMed]
- Haigh PI, Bilimoria KY, DiFronzo LA. Early postoperative outcomes after pancreaticoduodenectomy in the elderly. Arch Surg 2011;146:715-23. [Crossref] [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg 1998;2:207-16. [Crossref] [PubMed]
- Ito Y, Kenmochi T, Irino T, et al. The impact of surgical outcome after pancreaticoduodenectomy in elderly patients. World J Surg Oncol 2011;9:102. [Crossref] [PubMed]
- Scurtu R, Bachellier P, Oussoultzoglou E, et al. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg 2006;10:813-22. [Crossref] [PubMed]
- Hubbard RE, Story DA. Patient frailty: the elephant in the operating room. Anaesthesia 2014;69 Suppl 1:26-34. [Crossref] [PubMed]
- Kim SW, Han HS, Jung HW, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg 2014;149:633-40. [Crossref] [PubMed]
- Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg 2015;209:254-9. [Crossref] [PubMed]
- Kim SI, Duwayri Y, Brewster LP, et al. Frailty increases risk of mortality after elective abdominal aortic aneurysm (AAA) repair independent of age and comorbidity status. J Vasc Surg 2014;59:42S. [Crossref]
- McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant 2015;15:149-54. [Crossref] [PubMed]
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-8. [Crossref] [PubMed]
- Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology. J Am Geriatr Soc 2006;54:991-1001. [Crossref] [PubMed]
- Lee JS, He K, Harbaugh CM, et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg 2011;53:912-7. [Crossref] [PubMed]
- Hasselager R, Gögenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbecks Arch Surg 2014;399:287-95. [Crossref] [PubMed]
- Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345-52. [Crossref] [PubMed]
- Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg 2015;261:1173-83. [Crossref] [PubMed]
- Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. [Crossref] [PubMed]
- Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014;259:960-5. [Crossref] [PubMed]
- Sur MD, Namm JP, Hemmerich JA, et al. Radiographic sarcopenia and self-reported exhaustion independently predict NSQIP serious complications after pancreaticoduodenectomy in older adults. Ann Surg Oncol 2015;22:3897-904. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 2000;904:18-24. [Crossref] [PubMed]
- Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439-46. [Crossref] [PubMed]
- Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. [Crossref] [PubMed]
- Moons KG, Royston P, Vergouwe Y, et al. Prognosis and prognostic research: what, why, and how? BMJ 2009;338:b375. [Crossref] [PubMed]
- Cohen J, Cohen P, West S, et al. Applied Multiple Regression / Correlation Analysis for the Behavioral Sciences. 2nd ed. Hillsdale NJ Lawrence Erlbaum Assoc, 2003;Third Edit:703 S.
- House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 2008;12:270-8. [Crossref] [PubMed]
- Williams TK, Rosato EL, Kennedy EP, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg 2009;208:210-7. [Crossref] [PubMed]
- Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010;14:1143-50. [Crossref] [PubMed]
- Wolters U, Wolf T, Stützer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth 1996;77:217-22. [Crossref] [PubMed]
- Winter JM, Cameron JL, Yeo CJ, et al. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg 2007;204:1029-36; discussion 1037-8. [Crossref] [PubMed]
- Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. J Am Coll Surg 2013;216:976-85. [Crossref] [PubMed]
- Levi B, Lisiecki J, Zhang P, et al. Identifying important risk factors for surgical site infection in patients undergoing component separation ventral hernia repair through innovative analytic morphometric assessment and body composition. J Surg Res 2013;179:189. [Crossref]
- Waits S, Kim EK, Terjimanian MN, et al. Morphometric age and mortality after liver transplant. JAMA Surg 2014;149:335-40. [Crossref] [PubMed]
- Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 2015;22:2416-23. [Crossref] [PubMed]
- Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma : a new tool to assess sarcopenia. J Gastrointest Surg 2015;19:1593-602. [Crossref] [PubMed]
- Laugier R, Bernard JP, Berthezene P, et al. Changes in pancreatic exocrine secretion with age: Pancreatic exocrine secretion does decrease in the elderly. Digestion 1991;50:202-11. [Crossref] [PubMed]
- Ayus JC, Arieff AI. Abnormalities of water metabolism in the elderly. Semin Nephrol 1996;16:277-88. [PubMed]


