Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review
Introduction
Peritoneal mesothelioma (PM) is an uncommon but a serious, and often, fatal primary peritoneal tumour, with increasing incidence worldwide. As a primary peritoneal tumour, abdominal symptoms such as ascites, abdominal mass or intestinal occlusion, are the most frequent presentations (1). Different histological subtypes with different tumour aggressiveness have been described (2). Accurate histopathological analysis of an adequate biopsy specimen is needed when a primary peritoneal tumour is suspected. The pattern of spread of PM is predominantly expansive more than infiltrative or haematological. The presence of affected lymph nodes or extraperitoneal metastases are unusual, but when present, the prognosis is poor (3,4).
Systemic chemotherapy, generally based on experience with pleural mesothelioma, usually has disappointing results, even with novel chemotherapeutic agents (5-9). Evaluation of efficacy of systemic chemotherapy is difficult due to the low prevalence of the disease and difficulty of radiological assessment of response. As PM is confined to the abdomen for all, or much, of its clinical course, a multimodality treatment combining cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has emerged as a new standard of care with promising survival outcomes and local disease control in selected patients with PM.
This review updates the presentation, diagnosis, classification and treatment strategies for PM.
Aetiology and epidemiology of mesothelioma
Mesothelioma is an uncommon primary malignancy originating from mesothelial cells. The commonest site is in the pleural cavity, but 10–30% of all cases originate in the peritoneum (10) and less frequently in the pericardium, tunica vaginalis testis and ovarian epithelium (11). It has been estimated that 43,000 people worldwide die each year from mesothelioma (12). The global incidence is unknown but in the last three decades the incidence of mesothelioma has increased, with current estimates of more than 10,000 new cases per year in Australia, Japan, the USA and Western Europe (13).
Several epidemiological differences between pleural and PM have been reported. The median age at diagnosis is earlier in PM (63 vs. 71 years) (14), the incidence of cases not related to asbestos exposure is higher in the peritoneum and the latency period between asbestos exposure and development of mesothelioma is shorter (20 years in PM compared with 30–40 years in pleural) (15,16). Gender differences have also been reported. Pleural mesothelioma is more frequent in males and PM in women, often at a younger age than men (15). Likewise, prognosis seems better in females (17).
Asbestos exposure strongly correlates with an increased risk of pleural mesothelioma with a latency period in excess of 30 years. The link between asbestos exposure and PM is less strong, and it is estimated that approximately 20–40% of all PM cases occur spontaneously without previous asbestos exposure, especially in female patients (18,19). The mechanism whereby asbestos fibres reach the peritoneum is unknown but fibres have been found in the omentum and in the mesentery of the gastrointestinal tract (20). It is thought that irritation of the peritoneum induces a chronic inflammatory process, disruption of the mitotic process and chromosomal instability (21,22). Mesothelioma has also been described in relation to Mediterranean familial fever, germline mutations in BRCA genes, infection with simian vacuolating virus and chronic peritonitis (23).
Clinical presentation of PM
The clinical presentation of PM is comprised of a wide variety of mostly non-specific symptoms. The most frequently reported are abdominal pain and abdominal distension, occurring in more than 30–50% of patients (1,24). The more aggressive mesothelioma subtypes often present with rapid abdominal distension and intestinal obstruction due to a combination of large-volume omental disease and ascites. Other symptoms include weight loss, abdominal wall hernia, abdominal mass or anorexia (25-27). Often, mesothelioma is encountered incidentally, either on cross-sectional imaging or at abdominal laparoscopy or laparotomy (26). These non-specific symptoms may well lead to underestimation of the true incidence and late diagnosis.
Diagnosis
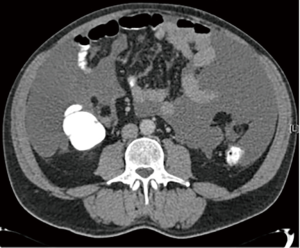
When PM is suspected, computed tomography (CT) of the chest, abdomen and pelvis is the initial imaging modality of choice (Figure 1). The administration of enteral contrast is recommended to delineate the small bowel and estimate the degree of small bowel involvement, which determines the feasibility of surgical options. A scoring system for small bowel and mesenteric involvement has been developed based on assessment by CT with positive enteral contrast (28). Magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT, have yet to demonstrate superiority over conventional CT in assessing small bowel involvement (29). Diagnostic laparoscopy is increasingly being used to better accurate the volume and distribution of the disease (30,31).
Confirmation of diagnosis requires histopathological analysis of tissue biopsies. Depending on the clinical situation, these biopsies may be obtained either percutaneously or surgically, preferably laparoscopically. Percutaneous aspiration and cytology of ascites alone has limited diagnostic potential and is not routinely recommended (2). The histological diagnosis of PM is based both on the morphology and immunohistochemistry. Mesothelioma typically stains positive for D2-40, cytokeratin 5/6 (CK 5/6), calretinin and Wilms tumour-1 (WT-1), and negative for BerEP4 antibody and thyroid transcription factor 1 (TTF1) (2). Recently, loss of expression of BRCA-associated protein 1 (BAP1) has been demonstrated to be highly specific in differentiating PM from (benign) mesothelial proliferation (32).
Serum CA-125 and CA 15-3 seem to have more of a role in monitoring recurrence than in establishing the initial diagnosis. Diagnostic sensitivity for CA-125 is 53% and 48.5% for CA 15-3 (33). Elevated CA-125 has been associated with epithelioid histology and massive peritoneal involvement (33). Other markers such as mesothelin and osteopontin show promise as potential markers, as they may be elevated in up to 71% of patients with PM with 84.6% sensitivity and 88.4% specificity (34,35).
Histological classification of PM
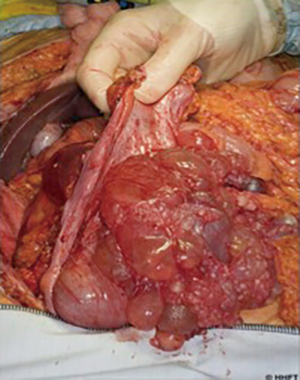
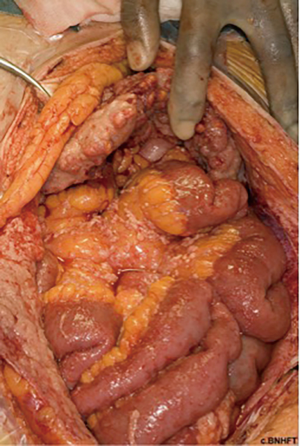
The term PM represents a spectrum of primary peritoneal tumours with varying degrees of malignant biology and clinical behaviour. At the lower end of this spectrum is multicystic mesothelioma (Figure 2), which is classified as a low-grade “borderline” malignant tumour that rarely metastasizes outside the abdomen but with high rates of locoregional recurrence (36). The more aggressive papillary variants likewise incorporate a spectrum from the more benign, well-differentiated papillary mesothelioma (WDPM), to diffuse malignant peritoneal mesothelioma (DMPM). WDPM is often grouped together with multicystic mesothelioma as a low-grade disease (37,38), although both disease variants have been reported to transform into more malignant subtypes (39). WDPM is more frequent in the peritoneum, compared with pleural variants, and by definition exhibits non-infiltrative growth patterns, in contrast to the aggressive papillary subtypes. DMPM is subdivided into epithelioid (the most frequent) (Figure 3), sarcomatoid and biphasic subtypes (40). Sarcomatoid and biphasic are generally highly aggressive tumours with rapid local progression, infiltrative growth patterns and lethal outcome.
Treatment
Systemic chemotherapy
The traditional treatment for PM has been systemic chemotherapy, using the same regimens developed for pleural mesothelioma (commonly a platinum-derivative combined with pemetrexed), supplemented, if necessary, with palliative debulking procedures to alleviate obstructive symptoms. Chemotherapy regimens included cisplatin and gemcitabine with a median survival of 6–9 months (41). Pemetrexed was the first agent approved for the treatment of advanced pleural mesothelioma. In a phase III trial, the combination of pemetrexed with cisplatin improved survival when compared with cisplatin alone, with response rates from 26% to 36% and a median survival of 12.1 months (5). Pemetrexed combined with gemcitabine as a first-line therapy demonstrated a 15% response rate, a better median survival period of 26.8 months but substantial toxicity (42). To date, systemic chemotherapy with, or without palliative surgery, has shown relatively poor response rates and low median survival of approximately 1 year (5). Novel agents, targeting mesothelin overexpression, are currently being developed for pancreatic, ovarian and gastric cancers and also for mesothelioma (43,44). In addition, phases I/II clinical trials evaluating the use of immunotoxin SS1P (45), chimeric anti-mesothelin antibody amatuximab (46) and mesothelin tumour vaccine CRS-207 (47), are ongoing.
CRS and hyperthermic intraperitoneal chemotherapy
As PM is a primary peritoneal malignancy generally confined to the abdominal cavity, locoregional treatment by a combination of CRS and HIPEC has been proposed. After the initial report of CRS and HIPEC in 10 PM patients confirmed technical efficacy, good palliation of ascites, and without treatment-related mortality (48), numerous reports have been published on this strategy for patients with PM (Table 1). A large multicentre review reports the outcomes of CRS and HIPEC in 401 patients with DMPM with a median overall survival of 53 months and 1-, 3- and 5-year survival rates of 81%, 60% and 47%, respectively (52). These survival outcomes are far superior to the 12–27 months median survival with systemic chemotherapy and best supportive care strategies, although no prospective, randomised studies have been performed directly comparing systemic chemotherapy with CRS and HIPEC.
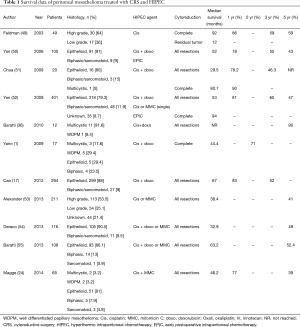
Full table
The main determinant of outcome after CRS and HIPEC is the completeness of surgical cytoreduction. The aim of surgery is a complete macroscopic tumour removal, achieved by a combination of peritonectomies and visceral resections. It has been suggested that an extensive “complete” parietal peritonectomy (i.e., removal of all peritoneum regardless of its macroscopic involvement at operation) is associated with better outcomes compared with peritoneal stripping of macroscopically affected peritoneum, as the risk of microscopic involvement of macroscopically normal peritoneum may be as high as 54% (56). After removal of all macroscopic disease, HIPEC is used to address microscopic disease. The most common HIPEC regimen for PM is a combination of cisplatin and doxorubicin for 60 minutes at 41–42°C, although significant variability between treatment centres exists. Early postoperative intraperitoneal chemotherapy (EPIC) could be administered after the CRS and HIPEC procedure, although evidence regarding this is contradictory (57-59). Estimated morbidity rates range between 28% and 41% for grade 3–4 complications, with perioperative mortality of approximately 1–2% (17,52-54). Major complications include haemorrhage, enterocutaneous fistula, perforation, dehiscence and abscess formation (52).
In patients in whom a complete cytoreduction is not deemed feasible, CRS and HIPEC may still be effective as a palliative procedure to manage symptoms and increase the likelihood of the patients commencing, and tolerating, systemic treatment. In these cases, a radical greater omentectomy, selected resections (frequently an extended right hemicolectomy or a subtotal colectomy) and/or stoma formation are combined with HIPEC to prevent rapid accumulation of ascites and to address intestinal obstruction. This strategy of maximal tumour debulking has resulted in survival benefits and improved symptom control in tumours presenting with malignant ascites (60-62) (Table 1).
The likelihood of achieving a complete cytoreduction depends on disease volume as well as distribution. Disease volume, commonly measured with the peritoneal cancer index (PCI), has been shown to be an independent predictor of outcome of CRS and HIPEC in other peritoneal malignancies (63-65), and can be estimated by preoperative imaging. Although all imaging has limitations for small lesions, certain radiological criteria have been reported to predict completeness of cytoreduction. Yan et al. identified the presence of a >5 cm tumour mass in the epigastric region, and the loss of normal architecture of the small bowel and its mesentery, as significant predictors: patients without these CT findings had a 94% probability of undergoing a complete cytoreduction (28). Nevertheless, imaging alone is often insufficient to exclude low volume or “miliary" small bowel disease. Staging laparoscopy is a useful mechanism in documenting disease extent and distribution, though requires general anaesthesia and has significant morbidity and a small mortality risk (40).
Several factors influence outcome after surgery in addition to disease extent and completeness of cytoreduction (Table 2). The administration of HIPEC has been demonstrated to be an independent predictor of improved survival, although all reports have been from retrospective studies where selection bias is likely (52). Limited data suggests that the choice of intraperitoneal chemotherapeutic agent may be of significance: in a cohort of 211 patients undergoing either mitomycin C or cisplatin-based HIPEC after complete CRS, significantly longer survival outcomes were demonstrated in the group treated with cisplatin (53). Epithelioid subtype has been identified as an independent predictor of increased survival when compared with biphasic and sarcomatoid (24,50,52,53,55). High preoperative levels of serum CA-125 are associated with adverse survival: 5-year overall survival rates for patients with normal (≤35 units/L) versus elevated CA-125 levels were 82% and 42.1%, respectively (33,66).

Full table
Gender has also been identified as a determinant of survival (17,50,52). In a multi-institutional registry of 294 patients with PM, significantly lower PCI was found in female patients and were also more likely to receive HIPEC. Overall survival rates in women undergoing CRS and HIPEC were higher than in men (1-, 3- and 5-year survival of 89%, 76% and 68%, versus 77%, 50%, and 39%, respectively) (17). Various hypotheses have been proposed to explain this observed difference between genders, such as variations in occupational asbestos exposure (67) as well as differences in tumour micro-environment and hormonal receptor expression. The role of the hormonal environment is further emphasised by the finding that postmenopausal women have a significantly worse survival outcome after CRS and HIPEC than younger, premenopausal women (17).
Other factors identified as independent predictors of improved survival after CRS and HIPEC include age <50 years (49,52,53), absence of lymph node metastases (17,50,55,56) and Ki-67 <10% (68).
A novel nomogram has been proposed to predict 3- and 5-year survival after CRS and HIPEC. In this model, histology, PCI at diagnosis and preoperative CA-125 levels were the three main factors affecting survival. This model had a positive and negative predictive value of 73.1% and 67.6%, respectively, at 3 years and of 73.9% and 73.3%, respectively, at 5 years (66).
The role of systemic chemotherapy in the context of CRS and HIPEC is controversial. In a study of 116 PM patients undergoing CRS and HIPEC, no survival differences were observed between those receiving pre- or post-operative chemotherapy compared with no chemotherapy, though substantial selection bias is likely to weaken the validity of comparison of these groups (43). A large multi-institutional study did not demonstrate any differences between different regimens and timings of systemic treatment (44).
In a recent multi-institutional retrospective study evaluating different chemotherapy strategies, patients who received systemic chemotherapy before CRS and HIPEC had shorter survival than those who has systemic chemotherapy after CRS and HIPEC (5). These results, and the lack of a good response rate to systemic chemotherapy (42,54) would suggest that in patients considered amenable to complete cytoreduction, upfront CRS and HIPEC should be considered rather than systemic chemotherapy. Nevertheless, some centres advocate neoadjuvant chemotherapy in all patients with PM, particularly as a “trial of time” strategy to further elucidate the biological behaviour of the tumour.
Conclusions
PM is a rare and challenging disease and should be included in the differential diagnosis of patients with peritoneal neoplasm. In highly selected patients with favourable histology, CRS and HIPEC offers a survival benefit over traditional treatment strategies consisting of palliative systemic chemotherapy. An accurate pre-operative histological classification and assessment of the distribution of the disease are crucial to select the patients who will benefit from this combination treatment of surgery and HIPEC. The role of systemic chemotherapy in the context of CRS and HIPEC (which drug and when to administer) in PM is unclear. Prognostic factors such as PCI, epithelioid subtype, absence of lymph nodes affected, complete cytoreduction and Ki-67 <10%, are well established as independent predictors of improved survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yano H, Moran BJ, Cecil TD, et al. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol 2009;35:980-5. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Yan TD, Deraco M, Elias D, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer 2011;117:1855-63. [Crossref] [PubMed]
- Bretagne CH, Petitjean A, Felix S, et al. Métastases révélatrices de mésothéliomes malins du péritoine : difficultés diagnostiques à propos de deux cas et conduite à tenir. Ann Pathol 2016;36:105-10. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma—Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [Crossref] [PubMed]
- Jänne PA, Wozniak AJ, Belani CP, et al. Open-Label Study of Pemetrexed Alone or in Combination with Cisplatin for the Treatment of Patients with Peritoneal Mesothelioma: Outcomes of an Expanded Access Program. Clin Lung Cancer 2005;7:40-6. [Crossref] [PubMed]
- Kelly RJ, Sharon E, Hassan R. Chemotherapy and targeted therapies for unresectable malignant mesothelioma. Lung Cancer 2011;73:256-63. [Crossref] [PubMed]
- Kepenekian V, Elias D, Passot G, et al. Diffuse malignant peritoneal mesothelioma: Evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE Database. Eur J Cancer 2016;65:69-79. [Crossref] [PubMed]
- Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol 1997;145:211-8. [Crossref] [PubMed]
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology 1997;30:403-18. [Crossref] [PubMed]
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-724C.
- McDonald JC, McDonald AD. The epidemiology of mesothelioma in historical context. Eur Respir J 1996;9:1932-42. [Crossref] [PubMed]
- Rodríguez D, Cheung MC, Housri N, et al. Malignant abdominal mesothelioma: defining the role of surgery. J Surg Oncol 2009;99:51-7. [Crossref] [PubMed]
- Spirtas R, Heineman EF, Bernstein L, et al. Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med 1994;51:804-11. [Crossref] [PubMed]
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13:405-12. [Crossref] [PubMed]
- Cao C, Yan TD, Deraco M, et al. Importance of gender in diffuse malignant peritoneal mesothelioma. Ann Oncol 2012;23:1494-8. [Crossref] [PubMed]
- Mensi C, De Matteis S, Catelan D, et al. Geographical patterns of mesothelioma incidence and asbestos exposure in Lombardy, Italy. Med Lav 2016;107:340-55. [PubMed]
- Mirarabshahii P, Pillai K, Chua TC, et al. Diffuse malignant peritoneal mesothelioma - An update on treatment. Cancer Treat Rev 2012;38:605-12. [Crossref] [PubMed]
- Dodson RF, O’Sullivan MF, Huang J, et al. Asbestos in extrapulmonary sites: omentum and mesentery. Chest 2000;117:486-93. [Crossref] [PubMed]
- Yang H, Bocchetta M, Kroczynska B, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A 2006;103:10397-402. [Crossref] [PubMed]
- Craighead JE, Akley NJ, Gould LB, et al. Characteristics of tumors and tumor cells cultured from experimental asbestos-induced mesotheliomas in rats. Am J Pathol 1987;129:448-62. [PubMed]
- Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 2013;34:1413-9. [Crossref] [PubMed]
- Magge D, Zenati MS, Austin F, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014;21:1159-65. [Crossref] [PubMed]
- Cao S, Jin S, Cao J, et al. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis 2015;30:1-10. [Crossref] [PubMed]
- Acherman YI, Welch LS, Bromley CM, et al. Clinical presentation of peritoneal mesothelioma. Tumori 2003;89:269-73. [PubMed]
- Manzini Vde P, Recchia L, Cafferata M, et al. Malignant peritoneal mesothelioma: a multicenter study on 81 cases. Ann Oncol 2010;21:348-53. [Crossref] [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 2005;103:839-49. [Crossref] [PubMed]
- Cao Q, Lu M, Heath J, et al. 18F-FDG PET/CT in a recurrent diffuse malignant peritoneal mesothelioma. Clin Nucl Med 2012;37:492-4. [Crossref] [PubMed]
- Valle M, Federici O, Garofalo A. Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy, and Role of Laparoscopy in Diagnosis, Staging, and Treatment. Surg Oncol Clin N Am 2012;21:515-31. [Crossref] [PubMed]
- Marmor RA, Kelly KJ, Lowy AM, et al. Laparoscopy is Safe and Accurate to Evaluate Peritoneal Surface Metastasis Prior to Cytoreductive Surgery. Ann Surg Oncol 2016;23:1461-7. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Baratti D, Kusamura S, Martinetti A, et al. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2007;14:500-8. [Crossref] [PubMed]
- Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006;12:447-53. [Crossref] [PubMed]
- Cristaudo A, Bonotti A, Simonini S, et al. Combined serum mesothelin and plasma osteopontin measurements in malignant pleural mesothelioma. J Thorac Oncol 2011;6:1587-93. [Crossref] [PubMed]
- Baratti D, Vaira M, Kusamura S, et al. Multicystic peritoneal mesothelioma: outcomes and patho-biological features in a multi-institutional series treated by cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Eur J Surg Oncol 2010;36:1047-53. [Crossref] [PubMed]
- Chen X, Sheng W, Wang J. Well-differentiated papillary mesothelioma: a clinicopathological and immunohistochemical study of 18 cases with additional observation. Histopathology 2013;62:805-13. [Crossref] [PubMed]
- Malpica A, Sant’Ambrogio S, Deavers MT, et al. Well-Differentiated Papillary Mesothelioma of the Female Peritoneum. Am J Surg Pathol 2012;36:117-27. [Crossref] [PubMed]
- González-Moreno S, Yan H, Alcorn KW, et al. Malignant transformation of benign cystic mesothelioma of the peritoneum. J Surg Oncol 2002;79:243-51. [Crossref] [PubMed]
- Laterza B, Kusamura S, Baratti D, et al. Role of explorative laparoscopy to evaluate optimal candidates for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal mesothelioma. In Vivo 2009;23:187-90. [PubMed]
- Fennell DA, Gaudino G, O’Byrne KJ, et al. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol 2008;5:136-47. [Crossref] [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: Final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [Crossref] [PubMed]
- Creaney J, Dick IM, Robinson BW. Discovery of new biomarkers for malignant mesothelioma. Curr Pulmonol Rep 2015;4:15-21. [Crossref] [PubMed]
- Einama T, Kawamata F, Kamachi H, et al. Clinical impacts of mesothelin expression in gastrointestinal carcinomas. World J Gastrointest Pathophysiol 2016;7:218. [Crossref] [PubMed]
- Kreitman RJ, Hassan R, Fitzgerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009;15:5274-9. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]
- Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858-68. [Crossref] [PubMed]
- Ma GY, Bartlett DL, Reed E, et al. Continuous hyperthermic peritoneal perfusion with cisplatin for the treatment of peritoneal mesothelioma. Cancer J Sci Am 1997;3:174-9. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [Crossref] [PubMed]
- Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2006;32:948-53. [Crossref] [PubMed]
- Chua TC, Yan TD, Morris DL. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: the Australian experience. J Surg Oncol 2009;99:109-13. [Crossref] [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- Alexander HR, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [Crossref] [PubMed]
- Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1093-100. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer 2013;49:3140-8. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416-24. [Crossref] [PubMed]
- Huang Y, Alzahrani NA, Liauw W, et al. Early Postoperative Intraperitoneal Chemotherapy for Low-Grade Appendiceal Mucinous Neoplasms with Pseudomyxoma Peritonei: Is it Beneficial? Ann Surg Oncol 2017;24:176-83. [Crossref] [PubMed]
- Lam JY, McConnell YJ, Rivard JD, et al. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg 2015;210:424-30. [Crossref] [PubMed]
- McConnell YJ, Mack LA, Francis WP, et al. HIPEC + EPIC versus HIPEC-alone: Differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol 2013;107:591-6. [Crossref] [PubMed]
- Dayal S, Taflampas P, Riss S, et al. Complete Cytoreduction for Pseudomyxoma Peritonei Is Optimal but Maximal Tumor Debulking May Be Beneficial in Patients in Whom Complete Tumor Removal Cannot Be Achieved. Dis Colon Rectum 2013;56:1366-72. [Crossref] [PubMed]
- Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J Surg Oncol 2009;100:331-4. [Crossref] [PubMed]
- Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 2008;34:154-8. [Crossref] [PubMed]
- Goéré D, Souadka A, Faron M, et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 2015;22:2958-64. [Crossref] [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [Crossref] [PubMed]
- Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123-31. [Crossref] [PubMed]
- Schaub NP, Alimchandani M, Quezado M, et al. A novel nomogram for peritoneal mesothelioma predicts survival. Ann Surg Oncol 2013;20:555-61. [Crossref] [PubMed]
- Welch LS, Acherman YI, Haile E, et al. Asbestos and peritoneal mesothelioma among college-educated men. Int J Occup Environ Health 2005;11:254-8. [Crossref] [PubMed]
- Kusamura S, Torres Mesa PA, Cabras A, et al. The Role of Ki-67 and Pre-cytoreduction Parameters in Selecting Diffuse Malignant Peritoneal Mesothelioma (DMPM) Patients for Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol 2016;23:1468-73. [Crossref] [PubMed]