|
Original Article
A new experimental model to allow use of clinical-scale endoscopes in small-animal tumor models
Mario Anders1, Eckart Frimberger2, Simone Odau3, Bertram Wiedenmann4, Thomas Rösch1
1Department of Interdisciplinary Endoscopy, University Hospital Hamburg Eppendorf, Hamburg, Germany; 2Department of Internal Medicine II, Technical University of Munich, Munich, Germany; 33Preclinics – Gesellschaft für präklinische Forschung mbH, Potsdam, Germany; 4Department of Internal Medicine, Divisions of Gastroenterology and Hepatology, Charité Medical School, Berlin, Germany
The study was supported by an unrestricted grant from Olympus Corp., Hamburg, Germany.
Corresponding to: Thomas Rösch, MD. Department of Interdisciplinary Endoscopy University Hospital Hamburg Eppendorf, Martinistr 52, 20246 Hamburg, Germany. Tel: +49-40-7410-50098; Fax: +49-40-7410-40004. Email: t.roesch@uke.de.
|
|
Abstract
Background: The evaluation of novel endoscopes may require testing in experimental tumor models, particularly when
employing new biomarkers. Tumor models, however, exist almost exclusively in small animals. Therefore, we aimed to
develop an experimental setting that allows the use of clinical-scale endoscopes in small animals.
Methods: In our approach, the proximal large bowel with intact blood supply is exposed on a movable and heightadjustable
table. The endoscope’s tip may be inserted into the bowel; the dark environment of the bowel lumen in vivo is
simulated by mounting a light-tight curtain around the endoscope. Proof-of-principle experiments were done in Wag/Rij
rats following cecal injection of the cell line R1H.
Results: Using high-definition television white-light endoscopy, narrow-band and autofluorescence imaging, and miniprobe-
based confocal laser microscopy (CLM) marked differences were observed between normal mucosa and tumors.
Depending on the techniques, mean examination times ranged from 3 to 10 minutes. Even after 90 minutes the colon
displayed an intact blood supply, imaged by Evans blue injection and by CLM.
Conclusion: These experiments demonstrate that our model allows in vivo examination of small-animals by clinicalscale
endoscopes. Therefore, it may be useful for evaluation, at various stages of GI carcinogenesis, of both new biomarkers
and endoscopic technologies.
Key words
abdominal malignancies, basic science, colon cancer, high-tech endoscopes, small-animal tumor models
J Gastrointest Oncol 2011; 2: 64-69. DOI: 10.3978/j.issn.2078-6891.2011.013
|
|
Introduction
Endoscopy has made enormous progress in imaging of
the gastrointestinal (GI) tract, ultimately leading to
better detection of luminal lesions, esp. in one of the
main areas such as screening colonoscopy for adenoma
detect ion. Never theless, neoplasia miss rates have repeatedly been reported, and the acceptance of classical
endoscopy as diagnostic and screening tool has limited
availability ( 1-4). Thus either better lesion detection
by conventional endoscopy and/or the introduction of
simpler and more patient-friendly techniques such as
capsule endoscopy may lead to better outcomes. With
simpler methods such as the colon capsule, a decrease in
sensitivity has to be accepted ( 5). This raises the need for
additional imaging technology using markers or other
red f lag techniques to overcome these limitations. Such
new markers will need to be tested in an experimental
live setting, as will new imaging technologies, in the
various stages of colon neoplasia as well as in tumors
elsewhere in the GI tract. Animal testing is usually required for any new technology
before being used in humans, particularly if they involve the
topical or intravenous (i.v.) application of new substances to
stain or highlight dysplastic or neoplastic lesions. However, tumor models have been preferentially developed in
rodents (mice and rats), while new endoscopes, particularly
prototypes, are manufactured exclusively with large
diameters (~9-12 mm) appropriate for patients. Current
attempts to address this issue include use of tumor models
in larger animals and the development and use of small (i.e.
rodent-scale) endoscopes but these have not yet progressed
significantly. Dedicated small-animal endoscopes, for
example, are often based on fiberoptic technology rather
than on high-resolution video, the technology routinely
used in clinical endoscopy ( 6, 7). The current study has therefore been undertaken to
combine both considerations, small-animal tumor models
and normal size clinical-scale endoscopes, in a single
experimental setting and to thereby establish a practical
method for evaluating new endoscopic techniques in smallanimal
models of colon carcinogenesis. The main aims
of the study were to determine (i) the feasibility of the
proposed method, and (ii) the duration of preservation of a
vital colon mucosa with intact blood supply under the study
conditions described.
|
|
Methods
Animal tumor model
The rhabdomyosarcoma cell line R1H, originally derived
from the jaw musculature of a WAG/Rij rat, was provided
by Dr. Annette Raabe, Department of Radiotherapy
and Radio-Oncology, University Hospital of Hamburg-
Eppendorf, Germany. Male WAG/Rij rats were purchased
from Charles River WIGA (Deutschland) GmbH (Sulzfeld,
Germany). All experiments were performed according to
German law at the Preclinics – Gesellschaft für präklinische
Forschung mbH (Potsdam, Germany).
For tumor formation, 50 μl of cell suspension of
2 x 107 R1H cells were injected at 4 sites into the cecal
wall of 12-week-old male WAG/Rij rats (n = 6) following
laparotomy under general anesthesia with isoflurane.
Another male WAG/Rij rat did not receive tumor cell
injections.
Intraoperative endoscopy
At 23 days after the cell injection, the animals underwent
a second laparotomy under inhalation anesthesia with
isoflurane. The cecum was then opened longitudinally. The
cut was made at the side contralateral to the insertion of the
mesenteric blood vessel, in order to preserve an unhindered
blood supply during the entire procedure. With the now
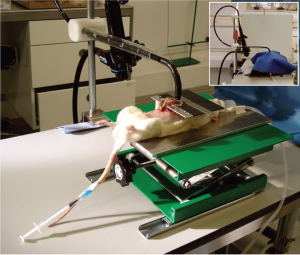
exposed luminal surface upwards, the cecum was positioned
on a specially designed height-adjustable table above the
animal’s chest; the table also had a measuring line alongside the exposed bowel.
The endoscope was fixed in an upright clamp with a screw
mechanism to allow adjustment of the distance between the
endoscope tip and the bowel (Fig 1). In order to simulate
the dark conditions of in vivo endoscopy, an opaque curtain
was fixed around the circumference of the distal portion of
the endoscope. Relative movement of the endoscope and
the bowel was achieved by slow and controllable movement
of the whole animal, placed on a movable hoist on top of
which was the small table for the exteriorized bowel, a unit
designed and built by E.F (Fig 1).
Following the procedure all animals were sacrificed
immediately using T61 (Intervet, Germany).
Endoscopes and confocal laser microscopy
For endoscopy, a Lucera CF-H260AZL/I® colonoscope
attached to the EVIS Lucera Spectrum® video system
(Olympus, Tokyo, Japan) was employed, providing 1080i
high-definition television (HDTV) imaging and two
additional light observation modes, that is, narrow-band
imaging (NBI) and autof luorescence imaging (AFI).
For confocal laser microscopy (CLM) a Leica animal
Z-probe attached to a laser scanning unit (Cellvizio®-Lab
system; Mauna Kea Technologies, Paris, France) was used.
Leica FCM 1000 IC software was employed for image
documentation. Immediately before the CLM examination,
0.1 ml of a 5% fluorescein solution (Alcon Pharma GmbH,
Freiburg, Germany) was injected into the rat’s tail vein.
|
|
Results
Preparation of the animal model
Sufficient inhalation anesthesia of an individual animal,
opening of the abdominal cavity, longitudinal exposure of
the cecum and cleansing of stool required a mean time of
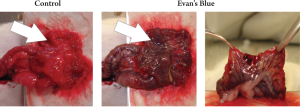
11 minutes (SD ±4 min). The integrity of the blood flow
was determined by i.v. administration of Evans blue dye
(Sigma-Aldrich, Munich, Germany) through the tail vein
of a tumor-free WAG/Rij rat, revealing an apparently intact
blood supply for the maximum tested time of 90 minutes
(Fig 2). The adequacy of the blood supply was also proven
by CLM imaging after i.v. administration of fluorescein in
all animals. Moreover, no notable bleeding occurred in any
of the animals.
The opened cecum, placed on a small table located above
the rat’s chest, offered an area of approximately 4 × 2 cm
of exposed mucosa for further endoscopic evaluation. All
animals that had undergone prior injection of the R1H
cell line displayed tumor nodes of 3–8 mm diameter at the
injection sites.
Assessment of NBI, AFI, and CLM in rat colon tumors
To test the applicability of a clinical-scale endoscope to
inspect the rat cecum, a colonoscope was placed close to
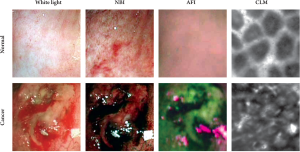
the mucosa. Conventional white-light endoscopy showed
that about two thirds of the cecum could be observed
while keeping a fixed position, with slight movements of the endoscope tip allowing gradual inspection of the
entire surface. Normal mucosa and tumor sites were clearly
distinguishable in all animals. The NBI mode enhanced
the visibility of the blood vessels and of the superficial
mucosal structure, displaying a regular structure in
untransformed areas. In contrast, tumor areas showed a
lack of structural organization. AFI clearly discriminated
normal mucosa and tumor, with sharp borders between
the purplish untransformed areas and the greenish tumors.
Subsequent CLM revealed distinctly different patterns for
normal mucosa, which displayed a honeycomb-like regular
structure, and for tumors, which showed an irregular
structure (Fig 3). Examination times depended on the
individual techniques: requiring 3 min (SD ± 30 s) for NBI
and AFI and 10 min (SD ± 2 min) for CLM.
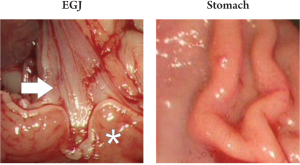
In addition, the procedure was terminated in one animal
by exposing the stomach and the esophagogastric junction
in a similar manner to the bowel; accessibility was proven,
but no further experiments with regards to blood supply or
prior tumor injection were performed in these areas (Fig 4).
|
|
Discussion
The objective of the current study was to establish a
practical and reliable method for evaluation of new
endoscopic imaging techniques, including biomarkers as
well as clinical scale endoscopes, in small-animal tumor
models. Rodent structures which can be imaged by the
method described include the colon, small bowel, stomach,
and esophagogastric juncture. Extension of this method
to capsule endoscopes and other such devices is readily
achievable as well.
Our experimental system requires two notable
components: first, a small table positioned over the animal
adjacent to the laparotomy wound to expose the animal’s
bowel properly; second, a curtain placed around the tip of
the endoscope in order to achieve light-tight examination
conditions simulating those in the GI lumen in vivo. The
assembly allowed relative movement, vertically and in all lateral directions, between the fixed endoscope and
the exposed bowel by movement of the whole animal on
a movable table. This setup proved to be adequate and
easy to use, requiring little time and effort for appropriate
positioning.
The newest endoscopic imaging strategies may require
topical or i.v. administration of substances to label
neoplastic cells or to mark cells and tissues, for example, the
use of fluorescein in confocal laser microscopy ( 8-10). The
evaluation of such substances may need testing in animals
with sustained intestinal vitality and intact blood supply.
Our data, using Evans blue dye for macroscopic inspection
and f luorescein for CLM (no CLM signal at all can be
detected if fluorescein is not delivered by the bloodstream),
suggest that the blood supply remains intact for at least
90 minutes when our setup is used. Therefore, it may be
suggested that this system offers a feasible technology to
test labeled (e.g. fluorescently marked) biomarkers. Given
the short examination times for each technique (3-10 min)
our model allows for evaluation of several methods in the
same animal. In addition, the model includes a very precise matching of imaging site and the site of histological
analysis
by using a measuring device alongside the exposed bowel.
Such a feature is particularly important in studying subtle
and perhaps macroscopically imperceptible lesions and/or
using so-called “endoscopic histology” techniques such as
CLM that sample only a very small area. A disadvantage of our model may be that sequential
examination of the same animal during various stages of
tumor development is not possible since intraoperative
endoscopy can only be per formed once. However,
sequential series of animals at different time intervals
after tumor induction may largely solve this problem.
Furthermore, in the same animal, precise identification of
the same site for follow-up endoscopy is difficult or even
impossible in any case.
In order to assess the new endoscopic technologies,
comparisons within def ined disease stages of colon
carcinogenesis are desirable. However, such conditions can
hardly be found in humans. Moreover, the comparison of
various techniques within an individual patient may be hard
to accomplish, as it may require a switch of endoscopes or
administration of several marker substances. Therefore,
tumor models resembling carcinogenesis in humans offer
a valuable tool for preclinical testing of endoscopes and
imaging technology. Several tumor models, including
knockdown of tumor suppressor genes, chemically induced
cancers, and orthotopic xenotransplantation of human
colon cancer cell lines have been developed ( 11, 12).
However, these models have been primarily established
in rodents that to date cannot be examined using clinicalscale
endoscopes. Our approach provides an opportunity
to employ these models to test such endoscopes. Thus
there is no requirement either for dedicated small-animal
endoscopes that are not adaptable to the full range of
available image-transmission technologies (since they are
fiberoptic-based) or for the time-consuming adaptation to
rodents of a particular clinical-scale endoscope to rodents
( 6, 7). As our experimental setting requires opening of
the intestinal lumen it may not be used to evaluate risks
of the endoscopic examination per se such as perforation.
However, it may help to reveal unwanted side effects of new
agents and/ or devices in terms of local tissue damage. In the cur rent proof-of-pr inciple study, we a lso
aimed to assess whether our approach might be used to
discriminate normal from malignant tissue. Therefore,
we tested conventional white-light endoscopy, NBI, AFI,
and also CLM, after intracecal injection of a sarcoma
cell line. Our results show that all these techniques
clearly distinguish areas of normal mucosa from tumors,
emphasizing that this approach could be used at various
stages of colon carcinogenesis. However, our data are based on a rhabdomyosarcoma cell line as this model had
been previously established and, therefore, offered an
immediate and well defined condition for the primary test
of our method. Moreover, as this cell line was originally
derived from WAG/Rij rats, as used for our experiments,
no particular considerations were necessary regarding the
immune status of the animals. Nonetheless, it must be stated
that due to the use of this cell line the extrapolation of our
results regarding tumor imaging to the conditions of colon
cancers is limited. In contrast to previous observations in
humans ( 13-15) with AFI we observed a purple signal in
normal areas whereas tumors appeared green. Potential
inter-species differences must therefore be considered in
evaluating new endoscopic technologies in non-human
systems. In summary, we have described a novel, practical method
for evaluation of new endoscopes and endoscopic imaging
technologies for the diagnosis of various GI cancers and their
precursors. Further studies of this method are currently
underway.
|
|
Acknowledgements
We thank Dr. Annette Raabe (Department of Radiotherapy
and Radio-Oncology, University Hospital, Hamburg-
Eppendorf) for kindly providing the R1H cell line.
|
|
References
- Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers
TE, et al. Predictors of nonadherence to screening colonoscopy. J Gen
Intern Med 2005;20:989-95.[LinkOut]
- Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for
colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci
2008;53:3049-54.[LinkOut]
- Pox C, Schmiegel W, Classen M. Cur rent status of screening
colonoscopy in Europe and in the United St ates . Endoscopy
2007;39:168-73.[LinkOut]
- van Rijn AF, van Rossum LG, Deutekom M, Laheij RJ, Bossuyt PM,
Fockens P, et al. Getting adequate information across to colorectal
cancer screening subjects can be difficult. J Med Screen 2008;15:149-52.[LinkOut]
- Eliakim AR. Video capsule endoscopy of the small bowel (PillCam SB).
Curr Opin Gastroenterol 2006;22:124-7.[LinkOut]
- Funovics MA, Alencar H, Su HS, Khazaie K, Weissleder R, Mahmood
U. Miniaturized multichannel near infrared endoscope for mouse
imaging. Mol Imaging 2003;2:350-7.[LinkOut]
- Funovics MA, Alencar H, Montet X, Weissleder R, Mahmood
U. Simultaneous f luorescence imaging of protease expression
and vascularity during murine colonoscopy for colonic lesion
characterization. Gastrointest Endosc 2006;64:589-97.[LinkOut]
- Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and
confocal microendoscopy. Nat Med 2008;14:454-8.[LinkOut]
- Odagi I, Kato T, Imazu H, Kaise M, Omar S, Tajiri H. Examination
of normal intestine using confocal endomicroscopy. J Gastroenterol
Hepatol 2007;22:658-62.[LinkOut]
- Zambelli A, Villanacci V, Buscarini E, Bassotti G, Albarello L.
Collagenous colitis: a case series with confocal laser microscopy and
histology correlation. Endoscopy 2008;40:606-8.[LinkOut]
- Nakagama H, Nakanishi M, Ochia iM. Model ing human colon
cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci
2005;96:627-36.[LinkOut]
- Vignjevic D, Fre S, Louvard D, Robine S. Conditional mouse models of
cancer. Handb Exp Pharmacol 2007;178:263-87.[LinkOut]
- Haringsma J, Tytgat GN, Yano H, Iishi H, Tatsuta M, Ogihara T, et al.
Autofluorescence endoscopy: feasibility of detection of GI neoplasms
unapparent to white light endoscopy with an evolving technology.
Gastrointest Endosc 2001;53:642-50.[LinkOut]
- Matsumoto T, Moriyama T, Yao T, Mibu R, Iida M. Autofluorescence
imaging colonoscopy for the diagnosis of dysplasia in ulcerative colitis.
Inflamm Bowel Dis 2007;13:640-1.[LinkOut]
- Wang CY, Lin JK, Chen BF, Chiang HK. Autofluorescence
spectroscopic differentiation between normal and cancerous colorectal
tissues by means of a two-peak ratio algorithm. J Formos Med Assoc
1999;98:837-43.[LinkOut]
Cite this article as:
Anders M, Frimberger E, Odau S, Wiedenmann B, Rösch T. A new experimental model to allow use of clinical-scale endoscopes in small-animal tumor models. J Gastrointest Oncol. 2011;2(2):64-69. DOI:10.3978/j.issn.2078-6891.2011.013
|