Continuum of care with anti-angiogenic therapies in metastatic colorectal cancer
Introduction
The number of available pharmacologic therapies for the systemic management of patients with metastatic colorectal cancer has grown at an impressive rate in recent years. For decades, 5-fluorouracil was the only available agent for the management of these cancers; between 2000 and 2012, an additional eight chemotherapies and biologic therapies were developed as potential options for their management. With this growth of options, an important consideration has been the best sequence and combinations of these agents’ use, so as to offer the longest clinical benefit to patients while minimizing the toxicities they experience.
An important class of agents within this expanded arsenal is the angiogenesis inhibitors. Angiogenesis, the process of new blood vessel formation, has been well established for its essential role in tumor growth and metastatic spread (1). The dominant factor controlling angiogenesis is VEGF, which consists of a family of six different proteins delineated as VEGF A through E, and PIGF (2). In cancer, the VEGF proteins function as ligands that bind to and activate three different receptor tyrosine kinases, thus activating a network of downstream signaling that promotes tumor angiogenesis (3). Thus, VEGF and the process of its receptor binding have proven to be important targets in the treatment of colorectal and other cancers.
The monoclonal antibody bevacizumab was the first approved therapeutic agent to target the process of angiogenesis in managing metastatic colorectal cancer (4). This antibody targets and binds VEGF-A, preventing its receptor binding and thus driving tumor angiogenesis (5). In addition to bevacizumab, two additional angiogenesis-targeting agents have been approved for the management of metastatic colorectal cancer.
Ziv-aflibercept has been approved for use with the chemotherapeutic regimen FOLFIRI for the management of metastatic colorectal cancer (6). Ziv-aflibercept acts as a soluble receptor, binding VEGF-A to VEGF-B and to PIGF, thus preventing these ligands from binding to and activating their receptors (7). The prefixed “ziv-aflibercept” is used to distinguish the use of aflibercept in the treatment of malignancy from its use in the treatment of macular degeneration, where unmodified “aflibercept” is used; for the remainder of this manuscript, as only the anti-tumor use of this agent will be addressed, “ziv-aflibercept” and “aflibercept” will be used interchangeably, and in accordance with the reference being discussed.
Regorafenib has been approved for the management of patients with metastatic colorectal cancer that have become refractory to all other therapeutic options (8). Regorafenib is an inhibitor of multiple angiogenic, stromal, and oncogenic kinases, including the VEGF receptors (9).
In this review, we present the evidence for the use of the available anti-angiogenic therapies in the management of metastatic colorectal cancer. The evidence for the use of these agents in the first-line, second-line, and refractory settings is reviewed, both for degree of clinical benefit as well as for associated adverse events. We present this evidence in the context of the chemotherapeutic regimens co-administered with these anti-angiogenic agents in the generation of these efficacy and toxicity data. Although good evidence for the use of each of these agents exists in certain lines of therapy for metastatic colorectal cancer, not all of their logical uses, in either the various lines of therapy or in combination with different agents, have yet been explored. There is little data yet about which of these anti-angiogenic agents might be superior to another when compared in a specific line of therapy, and on what biologic or demographic information may predict response to these agents. These gaps in data are noted when appropriate in order to develop a clear understanding of when and how the evidence supports the use of each anti-angiogenic agent in the management of metastatic colorectal cancer.
First line anti-angiogenesis therapy in metastatic colorectal cancer
In the first line management of metastatic colorectal cancer, bevacizumab is the only anti-angiogenic agent approved for use. Bevacizumab has been well studied in this setting, with good evidence for combining it with a number of different chemotherapeutic regimens, including fluoropyrimidine monotherapies, as well as combination regimens of a fluoropyrimidine and either irinotecan or oxaliplatin.
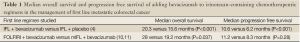
A survival benefit with bevacizumab in the management of metastatic colorectal cancer was first demonstrated with the addition of the antibody to the chemotherapeutic regimen IFL, which uses bolus 5-fluorouracil (4). Patients were randomized to receive IFL and either bevacizumab (5 mg/kg each cycle) or placebo. A statistically significant and clinically meaningful improvement in overall survival was observed among patients who received bevacizumab in addition to IFL when compared to those patients who received IFL with placebo. Statistically significant improvements with the addition of bevacizumab to IFL were also observed for the secondary endpoints of median duration of progression-free survival, response rate, and median duration of response; overall and progression free survival data are summarized in Table 1.
Safety and quality of life were also secondary endpoints in this study (4). As might be expected, the rates of a number of side effects that are associated with bevacizumab were higher in the treatment arm of the study when compared to the placebo arm, but these events were generally easily managed. These included grade 3 or 4 leukopenia (37% compared to 31.1%), grade 3 or 4 diarrhea (32.4% compared to 24.7%), hypertension (22.4% compared to 8.3%), thrombotic events (19.4% compared to 16.2%), grade 3 or 4 bleeding (3.1% compared to 2.5%), proteinuria (26.5% compared to 21.7%), and gastrointestinal perforation (1.5% compared to 0%). The rates of adverse events leading to death were equivalent, at 2.8% in the placebo arm versus 2.6% in the bevacizumab arm.
The bolus administration of 5-fluorouracil, such as in the IFL regimen, has fallen out of favor, due to the more palatable side effect profile of gastrointestinal toxicity that is associated with an infusional administration. Indeed, in the above study, patients who received IFL with placebo still had a grade 3 or 4 adverse event rate of 74% (4). Several studies have evaluated the combination of bevacizumab with an irinotecan-containing regimen with an infusional administration of 5-fluorouracil for the first line management of metastatic colorectal cancer.
One study, the BICC-C study, initially compared three different chemotherapeutic regimens that combined irinotecan with different methods of fluoropyrimidine administration in the front line management of metastatic colorectal cancer (10). Patients were randomized to these three different treatment arms, which consisted of FOLFIRI (which administers 5-fluorouracil as both a bolus and an infusion), mIFL (which administers 5-fluorouracil just as a bolus), and CapeIRI. During the study, the protocol was amended, after which time patients randomized to the FOLFIRI or mIFL arms of the study received bevacizumab as well; due to unacceptable toxicity levels, the CapeIRI arm was discontinued.
This amendment allowed for all patients going forward to receive bevacizumab in addition to their chemotherapy regimen, thus the study results cannot directly compare patients to receive chemotherapy with bevacizumab versus chemotherapy alone, even though there were patients enrolled under both circumstances at different points in the trial. In the FOLFIRI arm, bevacizumab was administered at 5 mg/kg with each 14-day cycle; in the mIFL arm, bevacizumab was administered at 7.5 mg/kg with each 21-day cycle.
For those patients treated prior to the amendment adding bevacizumab, a statistically significant difference in the primary objective of median progression free survival time was noted in the FOLFIRI arm over the mIFL arm (10). For those patients who were treated following the protocol amendment and thus received bevacizumab, the median progression free survival time was 11.2 months for patients treated with FOLFIRI and bevacizumab and 8.3 months for patients treated with mIFL and bevacizumab; the difference between these two arms, however, did not achieve statistical significance.
The secondary endpoint of median overall survival prior to the bevacizumab amendment resulted in a non-statistically significant difference of 23.1 months in the FOLFIRI arm versus 17.6 months in the mIFL arm (10). In a follow up of the BICC-C study, however, a statistically significant difference in median overall survival was noted when patients were treated with FOLFIRI and bevacizumab versus mIFL and bevacizumab (11). This survival was 28 months in the FOLFIRI plus bevacizumab arm versus 19.2 months in the mIFL plus bevacizumab arm. The bevacizumab survival data from BICC-C are summarized in Table 1.
Full Table
Toxicity was also a secondary endpoint in the BICC-C study and was reported in the initial publication (10). The grade 3 or greater toxicities associated with the use of bevacizumab with FOLFIRI were notable for a 12.5% hypertension rate; other toxicities occurred at rates similar to those seen with the use of FOLFIRI alone. The grade 3 or greater toxicities associated with the use of bevacizumab with mIFL were also notable for a hypertension rate of 1.7%, with other toxicities reported with occurrence rates somewhat lower than were seen with mIFL alone.
This information, taken together, demonstrates that a significant survival benefit was conferred by the use of FOLFIRI with bevacizumab compared to mIFL with bevacizumab in the first line management of patients with metastatic colorectal cancer, with an adverse event profile that generally could be easily managed. Although not designed to determine if the addition of bevacizumab to FOLFIRI is superior to FOFIRI alone in the initial management of metastatic colorectal cancer, the BICC-C study does establish the clinical benefit and tolerability of FOLFIRI with bevacizumab in the first line setting.
In addition to the combination of bevacizumab to irinotecan-containing regimens, a number of trials have evaluated the clinical benefit of adding bevacizumab to oxaliplatin-containing chemotherapy regimens in colorectal cancer in the first line, metastatic setting. The TREE-2 study evaluated the addition of bevacizumab to one of three different oxaliplatin-containing chemotherapeutic regimens (12). As was the case with BICC-C, the TREE study initially set out to investigate three different chemotherapy regimens without considering the added benefit of bevacizumab; the data prior to the addition of bevacizumab to the regimen are dubbed TREE-1 and those following its addition are referred to as TREE-2. The three chemotherapy regimens were mFOLFOX6 (which uses both bolus and infusional 5-fluorouracil), bFOL (which uses bolus 5-fluorouracil), and CapeOx. When bevacizumab was added to the treatment arms, it was administered on day 1 of each cycle, at doses of either 5 or 7.5 mg/kg depending on chemotherapy cycle length. As was the case with the BICC-C trial, the addition of bevacizumab allowed for all patients going forward to receive bevacizumab in addition to their chemotherapy regimen, thus this study does not directly compare patients receiving chemotherapy with bevacizumab to those receiving chemotherapy alone.
The primary end point of the TREE study was incidence of grade 3 or 4 treatment related adverse events during the first 12 weeks of therapy (12). As expected, several adverse events were reported with the use of bevacizumab in the TREE-2 data, with low calculated occurrence rates, which included bowel perforation, impaired wound healing, hypertension, and proteinuria.
Secondary endpoints of the TREE study evaluated clinical benefit, including response rates and median overall survival, which are summarized in Table 2 (12). The addition of bevacizumab to these chemotherapy regimens resulted in response rates and median overall survival durations of 52% and 26.1 months, 39% and 20.4 months, and 46% and 24.6 months, respectively. Based on the results of the TREE-2 study, the addition of bevacizumab to chemotherapy regimens combining oxaliplatin with fluoropyrimidines is well tolerated and clinically beneficial in the first line management of metastatic colorectal cancer.

Full Table
In addition to the TREE-2 study, the N016966 study also evaluated the addition of bevacizumab to first-line, oxaliplatin-based chemotherapy. In this study, however, the addition of bevacizumab to chemotherapy was planned from the onset, in order to evaluate the benefit of its inclusion (13). Patients were randomly assigned in a 2×2 analysis to receive a chemotherapeutic regimen of either XELOX or FOLFOX4 (which uses both bolus and infusion 5-fluorouracil). The patients were then randomized to receive either bevacizumab (at either 7.5 or 5 mg/kg depending on cycle length) or placebo.
A statistically significant improvement in the primary endpoint of progression free survival was noted when bevacizumab was added to one of these oxaliplatin-containing regimens (13). However, no statistically significant difference in overall survival resulted with the addition of bevacizumab, and the response rates were similar with or without the use of bevacizumab. These survival data are summarized in Table 2. The lack of overall survival benefit may be attributed to cessation of treatment prior to disease progression in many patients in this study; had it been continued to disease progression, a benefit may have been observed, as has been demonstrated in some analyses. Taking this criticism into account, and considering that the rates of adverse events related to the use of bevacizumab remained manageable, the use of bevacizumab in addition to an oxaliplatin-based first line chemotherapy regimen remains appropriate practice for the management for metastatic colorectal cancer.
The results of a phase II trial in patients aged 65 and above demonstrated that the addition of bevacizumab to 5-fluorouracil alone, without either irinotecan or oxaliplatin, was of added clinical benefit over 5-fluorouracil alone, in the first-line management of metastatic colorectal cancer (14). In this trial, all patients were assigned to receive chemotherapy consisting of leucovorin and bolus 5-fluorouracil, and were randomized to receive either bevacizumab (at 5 mg/kg with each cycle) or placebo. This study did not achieve a statistically significant improvement in median overall survival through the addition of bevacizumab to 5-fluorouracil. However, improvement in median progression free survival was significant. This suggests that the addition of bevacizumab to 5-fluorouracil in the first line management of metastatic colorectal cancer is a better option than 5-fluorouracil alone, for patients who cannot receive irinotecan nor oxaliplatin. The age of the patients in this trial suggests a tolerable, clinical utility for bevacizumab those in this older age demographic. These survival data are summarized in Table 3.

Full Table
Several oral tyrosine kinase inhibitors that block the various VEGF receptors including vatalanib, cediranib, and sunitinib have been evaluated in combination with chemotherapy in the first line management of metastatic colorectal cancer. None of these agents has demonstrated more clinical benefit to patients beyond what is seen with standard chemotherapy alone, or with the addition of bevacizumab to chemotherapy (15-17). A subgroup of patients with specific biologic markers, for example elevated LDH in the studies with vatalanib, was potentially identified that may benefit from these agents, which may be investigated in the future. In addition, these agents have consistently demonstrated a different toxicity profile than bevacizumab, for example with more diarrhea, nausea, and vomiting, suggesting a class effect specific to the tyrosine kinase inhibitors.
To date, the only anti-angiogenic agent with proven benefit in the first line management of metastatic colorectal cancer is bevacizumab. There are numerous options for its use, including in combination with oxaliplatin or irinotecan based regimens, or with 5-fluorouracil alone when neither of these other chemotherapies can be tolerated by the patient. The side-effect profile of the addition of bevacizumab to all of the various chemotherapeutic regimens has proven to be largely equivalent and reasonably managed relative to the demonstrated clinical benefit. Therefore, selection of the initial regimen used to manage metastatic colorectal cancer should be made with consideration for patient tolerability, with the decision to add bevacizumab based upon the independent consideration for the patient’s ability to tolerate its unique panel of adverse events in order to garner the clinical benefit associated to its combination with the selected regimen.
Second line anti-angiogenesis therapy in metastatic colorectal cancer
When patients with metastatic colorectal cancer progress through the first line of systemic chemotherapy, there are a number of well-studied roles for anti-angiogenesis agents included in their options for second line of therapy. As bevacizumab is increasingly used as a part of first line treatment regimens, an important question is whether it should be continued when synthesizing a second line treatment strategy. For patients who did not receive bevacizumab in the first line setting, the agent does have a role for initiation in a second line regimen. Additionally, ziv-aflibercept has a role in second line treatment regimens against colorectal cancer.
The use of bevacizumab, either alone or in combination with chemotherapy, for the second line treatment of patients with metastatic colorectal cancer who had not received it in the first line setting, was explored in the E3200 cooperative group study (18). Patients enrolled in this study all had progressed on a first line chemotherapy regiment that consisted of irinotecan and a fluoropyrimidine. They were randomized to treatment with either FOLFOX4 alone, bevacizumab alone, or the combination of FOLFOX4 and bevacizumab together. Of note, a higher dose of bevacizumab of 10 mg/kg was used in this trial than in the previous studies discussed.
A statistically significant improvement in the primary endpoint of overall survival was demonstrated when the combination therapy was compared to chemotherapy alone, with a lower median survival demonstrated among patients who received only bevacizumab (18). This statistically significant difference was also demonstrated in median progression free survival for patients who received combination therapy compared to patients who received chemotherapy alone, with a lower median progression free survival among patients who received bevacizumab monotherapy. Finally, response rates for patients receiving combination therapy were much higher than for patients who received either, chemotherapy alone or bevacizumab. Notable differences in rates of grade 3 or 4 adverse events that are associated with bevacizumab therapy between patients treated with combination therapy versus chemotherapy alone included hypertension, bleeding, and vomiting. These survival data are summarized in Table 4. The E3200 study demonstrates that bevacizumab added to FOLFOX4 in second line treatment of metastatic colorectal cancer improves survival, with controllable adverse events. It is not clear whether the higher dose of bevacizumab in this trial impacted clinical benefit or adverse event rates, but this dose difference should be noted and considered when administering bevacizumab in this setting.

Full Table
In addition to bevacizumab, proven clinical benefit via anti-angiogenic therapy in the management of metastatic colorectal cancer in the second line setting can be achieved with ziv-aflibercept. This was demonstrated by the VELOUR study (6). To be eligible for this study, patients had to have progressed on an oxaliplatin-based first line treatment regimen; they could not have received irinotecan previously, but prior bevacizumab was allowed. About 30% of patients had indeed been treated with prior bevacizumab. All patients were treated with FOLFIRI, and were randomized to receive either aflibercept (4 mg/kg each cycle) or placebo. A statistically significant improvement in median overall survival was noted with the addition of aflibercept to placebo, as well as in median progression free survival, although these translate into modest clinical benefits of 1-2 months. A number of adverse events in the aflibercept arm were comparable to those seen with bevacizumab, including bleeding, arterial and venous thromboembolic events, and proteinuria. However, the rates of grade 3 or 4 hypertension were 19.3% in the aflibercept arm, which is much higher than what has been observed using bevacizumab. Moreover, there were higher rates of nausea, vomiting, and diarrhea observed when aflibercept was combined with chemotherapy, which is not typically associated with bevacizumab in this setting. In the absence of a head-to-head trial evaluating efficacy of bevacizumab and aflibercept in this setting, consideration of this side-effect profile may prove to be the deciding factor for the use of either bevacizumab or aflibercept for the treatment of these patients’ cancers.
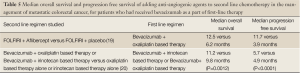
An important subset analysis from the VELOUR trial evaluated whether the use of bevacizumab with the oxaliplatin-based first line chemotherapy impacted the efficacy or tolerability of adding aflibercept to FOLFIRI in the second line management of metastatic colorectal cancer (19). In the initial trial, patients could enroll regardless of prior exposure to bevacizumab in the first line setting. This analysis took the exposure to bevacizumab into account, and found that, although not powered for survival, the use of bevacizumab in the first line of therapy did not impact clinical benefit of adding aflibercept to FOLFIRI in the second line therapeutic setting. These efficacy data are summarized in Tables 4,5. Among patients who were treated with aflibercept, the rates of grade 3 or 4 adverse events were similar between patients who received bevacizumab versus those who did not.
The issue of continuing bevacizumab in the second line setting when it has already been used in the first line management of metastatic colorectal cancer was focus of a European trial (20). Patients with metastatic colorectal cancer who had received first line treatment with bevacizumab plus chemotherapy that included either oxaliplatin or irinotecan were switched to the alternate chemotherapy, and then randomized to receive or not receive bevacizumab as well. A number of different chemotherapy regimens were used, but bevacizumab administration was consistent for those patients who were randomized to receive the agent. Using this treatment strategy, there were statistically significant improvements demonstrated by the addition of bevacizumab to both median overall survival and median progression free survival, which are summarized in Table 5. As might be expected from the first line use of bevacizumab with chemotherapy, there were slightly higher rates of grade 3 or greater adverse events in the bevacizumab arm versus the control arm, with four treatment related grade 5 events occurring in the bevacizumab arm and three events occurring in the control arm. The rates of benefit versus adverse events suggest that the continuation of bevacizumab along with second line chemotherapy is appropriate, even when it was a part of the first line treatment regimen.
Full Table
Importantly, the use of aflibercept has only been combined with the FOLFIRI chemotherapy regimen in the second line management of patients with metastatic colorectal cancer. Thus, for patients who have progressed through first line chemotherapy with an irinotecan-containing regimen, there is no evidence for the addition of aflibercept to an oxaliplatin-containing second line regimen. Should the use of an anti-angiogenic agent be desired in conjunction with an oxaliplatin-based second line chemotherapeutic regimen in metastatic colorectal cancer following progression on an irinotecan-based primary regimen, the studies described above demonstrate good evidence for the use of bevacizumab, regardless of whether or not it was a part of the primary therapeutic regimen (18,20). Thus far, there is no evidence for superior benefit or tolerance of either bevacizumab or aflibercept when added to chemotherapy in the second line management of metastatic colorectal cancer. Regardless of the first line chemotherapy used and of the use of bevacizumab first line, there is good evidence for the inclusion of an anti-angiogenic agent in conjunction with second line chemotherapy in this patient population, with the specific selection of the anti-angiogenic agent to be dictated by the chemotherapeutic regimen to be used and the potential side-effects associated with the different anti-angiogenic agents.
Anti-angiogenesis therapy in refractory metastatic colorectal cancer
As the role for the various anti-angiogenesis agents has been explored in a variety of settings, bevacizumab has been evaluated in an expanded access trial for activity in patients who had progressed through all standard chemotherapy but remained bevacizumab naïve. In this single arm study, patients whose metastatic colorectal cancer was refractory to irinotecan and oxaliplatin containing chemotherapy regimens were treated with a combination of bevacizumab, and leucovorin/5-fluorourical (either as bolus or continuous infusion at the treating physician’s discretion) (21). An important restriction in this trial was that patients could not have received bevacizumab previously. A response rate of 4% resulted, with side effect rates similar to other trials. This suggests that bevacizumab does retain clinical benefit for the management of patients whose cancers have become refractory to multiple other lines of therapy.
For patients with metastatic colorectal cancer who have progressed beyond all other approved standard systemic therapies, regorafenib has proven clinical benefit. This was demonstrated in the CORRECT study (8). Patients had to have received treatment including a fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and, for patient who had a Kras wild-type tumor, cetuximab or panitumumab. Patients were randomized to receive either regorafenib 160 mg by mouth once daily, for days 1-21 of a 28 day cycle, or a placebo. A statistically significant, marginal clinical benefit of 1.4 months of overall survival was observed in the regorafenib arm compared to placebo. Response rates were low in both trial arms and did not achieve statistical significance, but disease control rates were significantly higher in the regorafenib arm. Notably, regorafenib is the first agent with activity as a VEGF-receptor tyrosine kinase inhibitor to have benefit in metastatic colorectal cancer, whereas a number of other such agents have failed, as previously described. Given the wider range of tyrosine kinases that regorafenib inhibits, it is not clear whether this clinical benefit of regorafenib is attributable to its anti-VEGF activity or to another of its targets.
For this survival benefit in the CORRECT trial, 54% of treatment patients experienced grade 3 or 4 adverse events, compared to 14% experienced by patients in the placebo arm (8). Adverse events of grade 3 or 4 that occurred notably higher in the treatment arm when compared to the control arm included hand/foot syndrome, fatigue, diarrhea, hypertension, and rash. On the basis of the CORRECT study, regorafenib has garnered approval for patients with metastatic colorectal cancer who have progressed beyond all other available standard therapies. Presently, there is no approved role for this agent, outside of a clinical trial, in patients who still have other approved options available for the treatment of their metastatic colorectal cancer.
Conclusions
Anti-angiogenic agents have emerged as an important tool in the management of patients with metastatic colorectal cancer, in all lines of therapy, and in conjunction with a number of different chemotherapy regimens. Bevacizumab has applications in the first and second lines of metastatic therapy and remains the only anti-angiogenic agent approved in the first line setting. Ziv-aflibercept has also demonstrated a survival benefit in the second-line setting, in combination with chemotherapy. The anticancer activity demonstrated with regorafenib in the third line (or beyond) setting, even after prior anti-VEGF therapy demonstrates that there is a role and benefit for anti-angiogenic therapy throughout the continuum of care for patients with metastatic colorectal cancer, and that benefit may be seen with different agents, which target different parts of the angiogenic process.
As first line therapy for metastatic colorectal cancer, bevacizumab can be combined with both irinotecan and oxaliplatin containing regimens (4,10-13). Currently, there is no data to suggest that combining bevacizumab with any one regimen will have improved clinical benefit or tolerability in this setting, and therefore, the decision for a first line regimen should be based upon an individual patient’s anticipated tolerability to an adverse event profile. For those patients who cannot tolerate oxaliplatin or irinotecan, there is some evidence for administering bevacizumab with 5-fluorouracil alone, which was generated from an exclusively older patient population (14).
Although there are well-documented increases in bevacizumab-associated adverse events with each chemotherapy combination evaluated, these are relatively equivalent with each combination, and have never been studied for direct comparison. Certainly, the ability to tolerate bevacizumab should be a consideration for the addition of it to a conventional chemotherapeutic regimen, although it is noted throughout the studies that most of the bevacizumab associated adverse events are manageable.
Once metastatic colorectal cancer has progressed through a first line treatment regimen, there is ample evidence supporting the inclusion of an anti-angiogenic agent as a part of a second line therapy. This is based on survival and response benefits with an acceptable toxicity profile, and has been demonstrated both for the treatment of patients whose first-line regimens included bevacizumab as well as for those whose did not. For patients whose cancers were treated first line with bevacizumab and either irinotecan or oxaliplatin, there is strong evidence supporting the use of bevacizumab in conjunction with a second-line chemotherapeutic regimen that includes the alternate agent (18,20).
Moreover, for patients whose initial chemotherapy was oxaliplatin based, there is good evidence for the use of ziv-aflibercept in conjunction with an irinotecan-based second line therapy (6). Subset analysis suggests that this benefit holds true both for patients who received bevacizumab as a part of their oxaliplatin-based first line therapy as well as for those patients who did not, although the study was not powered to determine if this benefit was statistically significant (19). The use of ziv-aflibercept in the second line therapy of patients who are irinotecan refractory and thus receive oxaliplatin in the second line has not been established, and thus this combination should not be used outside of a trial setting, particularly considering that bevacizumab can be used in this setting, and with a fairly comparable adverse event rate and profile.
Anti-angiogenic therapies remain an important component of the treatment of patients who have progressed and become refractory to other available standard first line agents. There is good evidence for the addition of bevacizumab to 5-fluorouracil in the management of such patients who are refractory to both oxaliplatin and irinotecan, but this has only been explored in patients who did not receive bevacizumab in earlier regimens. Given the data presented above, it would be unusual for patients to remain bevacizumab naïve at this time (21). Perhaps more clinically applicable is the use of regorafenib in the management of patients who have become refractory to all other therapeutic options, including bevacizumab (8).
As we go forward, important questions will address how to individualize the selection of the various anti-angiogenic agents for inclusion or exclusion in the therapeutic management of metastatic colorectal cancer. Thus far, increased patient age does not appear to increase adverse event rate associated with bevacizumab (14). This does not consider, however, whether the impact of these adverse events, when they do occur, result in an increased morbidity or mortality after a certain age. Regardless of age, another important consideration will be whether certain adverse events associated with the use of these agents preclude their repeated use. For instance, it is unclear whether a patient who has experienced a bowel perforation on bevacizumab in the first line setting (which is currently the most likely scenario as it has been approved for nearly a decade) should preclude the use of other anti-angiogenic therapies in the future. Other ways to select for patients who are more likely to benefit when their cancers are treated with these anti-angiogenic agents may evolve from within their tumors’ biology. There may be a role for measuring or identifying different biomarkers that would indicate a higher expected benefit from these agents. Such information would further guide the decision to include these agents when weighed against the risk posed to that individual by the agents’ adverse event profile.
As each of these agents’ effectiveness and tolerability in each line of therapy and in the context of the individual patient characteristics becomes clear as separate entities, it will be important to evaluate if any of these agents are more effective and/or more tolerable than another in each setting, through trials that directly compare them to one another. Until the efficacy and tolerability of each of these agents is understood in each line of therapy and with each possible chemotherapeutic combination, employment of each agent should be limited to the indications that they are presently assigned, based upon the available benefit and tolerance data. Fortunately, while the remaining questions are being explored, there are a variety of different options for the use of anti-angiogenic treatment in all lines of therapy in metastatic colorectal cancer.
Acknowledgements
Disclosure: Dr Hwang is a consultant and speaker for Roche/Genentech, Bayer and Onyx; Dr Smaglo declare no conflict of interest.
References
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [PubMed]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [PubMed]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Avastin. South San Francisco, CA: Genentech, Inc. and the Roche Group, 2013.
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [PubMed]
- Zaltrap. Bridgewater. NJ: Sanofi-Aventis US, LLC, 2012.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Stivarga. Wayne. NJ: Bayer HealthCare Pharmaceuticals Inc, 2013.
- Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779-86. [PubMed]
- Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol 2008;26:689-90. [PubMed]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 2008;26:3523-9. [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005;23:3697-705. [PubMed]
- Hecht JR, Trarbach T, Hainsworth JD, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:1997-2003. [PubMed]
- Hoff PM, Hochhaus A, Pestalozzi BC, et al. Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX in patients with previously untreated metastatic colorectal cancer: a randomized, double-blind, phase III study (HORIZON II). J Clin Oncol 2012;30:3596-603. [PubMed]
- Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol 2013;31:1341-7. [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [PubMed]
- Allegra CJ, Lakomy R, Tabernero J, et al. Effects of prior bevacizumab (B) use on outcomes from the VELOUR study: A phase III study of aflibercept (Afl) and FOLFIRI in patients (pts) with metastatic colorectal cancer (mCRC) after failure of an oxaliplatin regimen. J Clin Oncol 2012;30:abstr 3505.
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29-37. [PubMed]
- Chen HX, Mooney M, Boron M, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol 2006;24:3354-60. [PubMed]


