An elderly man with remote history of metastatic melanoma now with localized pancreas cancer and new liver masses
Dr. Vasan: This is the case of an 84-year-old man who presented to his primary care physician with abdominal discomfort and diarrhea. Esophagogastroduodenoscopy (EGD) was unremarkable. Computed tomography (CT) of the abdomen and pelvis and magnetic resonance cholangiopancreatography (MRCP) were obtained. Serum tumor markers were not available.
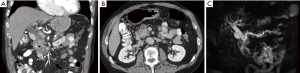
Dr. Do: On the coronal image (Figure 1A) we see biliary ductal dilatation interrupted at the level of the pancreatic head, and on the axial image (Figure 1B) a relatively normal appearing pancreatic head. On MRCP (Figure 1C) you can see a double duct sign which is a dilated pancreatic duct and dilated biliary tree. The double duct sign is almost pathognomonic for malignancy in the pancreatic head or ampulla (1). The absence of a discrete radiographic mass may be a good prognostic sign given that the mass may be small enough for resection. This usually leads to endoscopic ultrasound or sometimes directly to surgery.
Dr. O’Reilly: Dr. Do, could you please comment on the lymph nodes and the vasculature?
Dr. Do: No lymphadenopathy is noted. On the axial view (Figure 1B) there is a complete fat plane between the superior mesenteric vein (SMV) and the putative pancreatic head mass. There is also no involvement of the celiac artery or the superior mesenteric artery (SMA).
Dr. Vasan: The patient underwent endoscopic ultrasound and fine needle aspiration which demonstrated an ampulla with a fish mouth deformity and mucin consistent with an intrapapillary mucinous neoplasm (IPMN). Fine needle aspiration fluid showed CEA of 196 ng/mL and amylase greater than 183,000 units/L. Cytology showed suspicious cells, likely IPMN.
Dr. Vasan: The patient’s past medical history was significant for remote metastatic melanoma. Twenty-six years before, he developed a chest wall lesion and underwent resection. Pathology showed melanoma. He underwent bilateral prophylactic axillary dissection which demonstrated five lymph nodes positive for melanoma. He received an adjuvant vaccine. Four months later, he had surveillance scans which showed metastatic liver lesions. It was not clear if he had a liver biopsy. He was treated with dacarbazine, cisplatin, carmustine, and tamoxifen based on the Dartmouth protocol (2). He had a complete response. He was continued on maintenance dacarbazine and tamoxifen for five years and had no evidence of disease. Thereafter he was monitored expectantly. His other history was significant for atrial fibrillation, superior mesenteric artery thrombus, and gastroesophageal reflux disease. He had a prior cholecystectomy for cholelithiasis. He is a neversmoker. He is of Ashkenazi Jewish ethnic descent.
Dr. Abou-Alfa: Dr. Shoushtari, thanks for joining us. Could you comment on his melanoma management?
Dr. Shoushtari: The Dartmouth protocol is combination cytotoxic regimen developed at Dartmouth University in the 1980s (2). Like many cytotoxic regimens in melanoma, it is associated with a 10–15% response rate. A phase III trial did not show an overall survival benefit as compared to standard single agent dacarbazine (3). However, he had a complete response which is quite unusual. We should note that he received alkylating agents as we think about why he may have had a complete response for his melanoma.
Dr. O’Reilly : A fascinating part of the presentation is the constellation of melanoma and pancreatic cancer, which is associated with germline p16 (CDKN2A) mutations and also BRCA mutations (4). He is Ashkenazi Jewish which raises the possibility of an underlying germline BRCA mutation.
We have been routinely genotyping patients with an in-house sequencing platform called MSK IMPACT (5). We have picked up ~15% germline genetic mutations predisposing to pancreatic cancers in patients thought to be high risk (6). What is the current global practice for testing for germline genetic mutations?
Dr. Shamseddine: At the American University of Beirut, we can test for germline BRCA mutations and microsatellite instability (MSI) in our breast and colon cancer patients, and we have identified patients and families with both. In Lebanon we have low rates of melanoma and do not routinely test for genetic mutations. Nonetheless, we are building a genetic counseling program for patients with multiple cancers.
Dr. Saltz: This raises an important and complicated question that is raised for many cancer patients. When is it standard practice in pancreas cancer and melanoma to perform formal genetic screening and when is it not? There are no specific criteria from any scientific body in that regard. From a research point of view, we commonly sequence pancreatic tumors at our institution but this is not standard practice. Recent retrospective data were interpreted to infer that all young patients with a new colorectal cancer diagnosis should be referred for genetic testing (7). However, on closer inspection none of the group of young colorectal cancer patients without mismatch repair deficiency or clinical features for a germline genetic syndrome had evidence of a germline genetic abnormality (8). Thus there would be no change in outcomes. The utility of these tests still remains debated. Furthermore it is not clear what clinicians should do with this information.
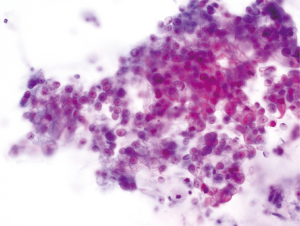
Dr. Vasan: The patient developed jaundice, dark urine, acholic stool, and pruritus. He had laboratory tests which showed AST 290 units/L, ALT 170 units/L, Alk Phos 1,866 units/L, total bilirubin 13.1 mg/dL, direct bilirubin 10.2 mg/dL, amylase 205 units/L, lipase 268 units/L, INR 7.1, CA 19–9 18 units/mL, CEA 2.0 ng/mL, WBC 5,100 cells/uL, Hb 13.9 g/dL, Plt 172,000 cells/uL. He underwent an endoscopic retrograde cholangiopancreatography (ERCP) which demonstrated pancreatic ductal dilatation. A stent was placed in the pancreatic duct and a biliary sphincterotomy and common bile duct (CBD) stent placement was performed. Brushings were obtained from the pancreatic duct and CBD and cytology from the pancreatic duct showed cells suspicious for adenocarcinoma. He underwent an endoscopic ultrasound (EUS) with fine needle aspiration (FNA). Cytology showed adenocarcinoma (Figure 2).
Dr. Abou-Alfa: Dr. Edelweiss, could you comment on the cytology?
Dr. Edelweiss : Cytology showed a group of cells arranged in a disordered glandular pattern with marked variation in nuclear size typical of pancreatic adenocarcinoma.
Dr. Vasan: He subsequently underwent a pancreaticoduodenectomy. Pathology showed a 4.8 cm moderately differentiated pancreatic head ductal adenocarcinoma. There was extrapancreatic invasion into the peripancreatic soft tissue, common bile duct (CBD), and duodenal wall. There was no lymphovascular invasion. There was perineural invasion. The pancreatic resection margin was positive. 0/23 lymph nodes were positive for carcinoma. He was staged as pT3N0 stage IIA with R1 resection.
His physical exam was unremarkable with Eastern Cooperative Oncology Group (ECOG) performance status 1.
Dr. O’Reilly: What are the standard options for chemotherapy for the adjuvant treatment of pancreas cancer?
Dr. Braghiroli: The standard options are adjuvant gemcitabine and 5-fluorouracil (5-FU). Gemcitabine is preferred given its favorable side effect profile. CONKO-001 (9) compared adjuvant gemcitabine versus observation and showed improvement in disease-free (13.4 vs. 6.7 months) and overall survival (22.8 vs. 20.2 months). Of note, the 5-year overall survival was 20.7% vs. 10.4% and the 10-year overall survival was 12.2% vs. 7.7%. ESPAC-3 (10) established that 5-FU with leucovorin is non inferior to gemcitabine regarding overall survival (23.0 vs. 23.6 months).
Dr. O’Reilly: It is important to note that in these trials 5-FU was given per the older Mayo Clinic regimen (11), which may explain the higher rate of side effects for fluoropyrimidine therapy in these older studies.
The landscape of adjuvant pancreas cancer treatment is changing as we try to bring regimens effective in the metastatic setting into the adjuvant setting. ESPAC-4 (12) investigated whether adjuvant gemcitabine with capecitabine improved survival as compared to adjuvant gemcitabine alone. Patients received six 4-week cycles of gemcitabine with or without capecitabine. The primary endpoint was overall survival. The trial met its primary endpoint with median overall survival of 28.0 vs. 25.5 months. Of note, ESPAC-4 was reported after this patient was received adjuvant therapy. Other ongoing trials are APACT (13) which is investigating adjuvant gemcitabine with or without nab-paclitaxel; and PRODIGE 24/ACCORD 24 (14) which is investigating adjuvant gemcitabine versus 5-FU with leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX).
Dr. Vasan: The patient was evaluated by medical oncology and was recommended adjuvant gemcitabine. Surveillance imaging after four cycles showed no evidence of disease. He received two additional cycles of gemcitabine and completed six total cycles. He underwent adjuvant chemotherapy and radiation with capecitabine used as a radiation sensitizer.
Dr. O’Reilly: The data on adjuvant chemoradiation remain controversial. Older data suggested an overall survival benefit. Follow-up studies, most notably ESPAC-1 (15) showed that adjuvant radiation was not associated with survival benefit as compared to adjuvant chemotherapy (15.9 vs. 17.9 months, P=0.05) and may be associated with decreased overall survival because patients may have been delayed in receiving systemic chemotherapy. RTOG 0848 (16) is an ongoing study designed to answer definitively the question of adjuvant radiation after adjuvant gemcitabine in pancreas cancer. I favored and recommended that this patient receive adjuvant radiation given his positive margins. Can our colleagues from the American University of Beirut (AUB) and National Guard Hospital in Riyadh comment on your use of chemoradiation?
Dr. Shamseddine: Our standard practice is adjuvant chemotherapy with gemcitabine. We have ceased using adjuvant chemoradiation, in view of the referenced data of ESPAC-1.
Dr. Olayan: We concur, nonetheless I understand the argument Dr. O’Reilly brings in to use radiation in this specific situation.
Dr. O’Reilly: The issue of margin assessment in pancreas cancer is controversial and can be influenced by specimen orientation and evaluation and surgical/pathology handling. Data regarding the efficacy of radiation in patients with positive margins are mixed, and ESPAC-1 suggested that the patients with T3 or T4 disease, positive margins, or nodal involvement received the most benefit from adjuvant chemoradiation (15).
Dr. Vasan: CT chest, abdomen, and pelvis 8 months following completion of adjuvant therapy revealed new liver lesions and bilateral subcentimeter pulmonary nodules. Laboratories showed stable CA 19–9 of 13 units/mL, stable CEA of 1.6 ng/mL, and an increased LDH from 268 to 551 units/L.
Dr. Do: There are two ill-defined liver lesions (Figure 3A,B) that are faintly hypoattenuating and a single well-circumscribed subcentimeter lung nodule (Figure 3C). This pattern is atypical for metastatic pancreas cancer where we commonly see more irregularly hypovascular liver lesions and several irregular pulmonary nodules rather than a single circumscribed nodule.
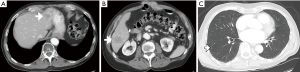
Dr. O’Reilly: Liver metastases are common in pancreatic cancer. However, his quick relapse within one year for a node-negative tumor was somewhat unusual. Also, the tumor markers remained stable and not elevated which also may not support this possibility of recurrence. It would not be unreasonable to assume that this is metastatic pancreas cancer but given his history and the unusual clinical features, we recommended a liver biopsy.
Dr. Shamseddine: I add considering the patient’s history of melanoma with low volume node-negative disease I would favor a biopsy.
Dr. Do: Sometimes MRI of the abdomen can help distinguish metastatic melanoma from other liver metastases, given the often intrinsic high T1 signal of melanoma metastases, a relatively unique feature.
Dr. Abou-Alfa: How often do you perform liver biopsy in patients without history of other malignancies?
Dr. Saltz: The conventional older teaching of re-biopsy with every first recurrence to prove malignancy was likely borne out of the poorer resolution of older radiographic modalities. Current modalities can often distinguish between metastases and non-metastases. I envision three scenarios: MRI of the abdomen, rescanning after a short interval, and biopsy. However the overall picture of his markers and atypical liver lesions make me favor biopsy.
Dr. Vasan: The patient underwent a liver biopsy. Pathology showed malignant melanoma with extensive necrosis (Figure 4A). Immunohistochemistry showed positive staining for HMB45 (Figure 4B), and A103 (Figure 4C).
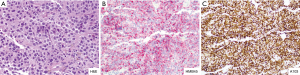
Dr. Shoushtari: This is a very unusual presentation of metastatic melanoma given his prior complete response with an extremely long period of remission. The highest rate of recurrence for stage IIIC melanoma is within the first two years and the rate of recurrence per year decreases to less than 5% after two years (17). Given these findings we do not routinely perform surveillance scans after two years for patients with stage IIIC melanoma out of risks for low yield and scan anxiety and rely on symptoms to warrant additional testing.
Dr. Vasan: The patient underwent intracranial imaging for melanoma staging, which was unremarkable. To guide therapeutic options, the patient underwent somatic testing on the liver metastasis and also germ-line genetic testing. The liver lesion identified a BRAF V600E mutation. There was a TERT (telomerase gene) promoter variant observed. There were also genetic alterations of unknown significance including TP53 R181C (TP53 is a known tumor suppressor), RIT1 amplification, ABL1 V722I, GRIN2A R1067W, MLL3 G2136E, NCOR1 T574I, NOTCH3 P197L, and STAG2 P987S. He also underwent germline genetic testing which showed a BRCA2 C9466T transition, which codes for a nonsense mutation resulting in loss of function of the tumor suppressor BRCA2.
Dr. O’Reilly: This genetic analysis further corroborates that this is not metastatic pancreatic cancer where we commonly see mutations in KRAS, p53, CDKN2A, and SMAD4 (18). His BRCA2 mutation is not one of the canonical Ashkenazi Jewish founder mutations and is associated with Spanish and Mediterranean ethnicities (19).
Dr. Shoushtari: About 70% of metastatic melanomas have variant in the TERT promoter gene (20). Dr. O’Reilly, how do we currently target RAS mutations in pancreatic cancer?
Dr. O’Reilly: About 30% of patients with pancreatic cancer have potentially “actionable” mutations as defined by several guideline criteria. These criteria however, indicate that a given finding has been identified to be treatable with a certain therapeutic and do not necessarily indicate that targeting with a certain drug in a certain pathway has been shown to be of benefit in the particular disease under evaluation. In pancreas cancer the most common findings of actionability relate to the DDR (DNA–damaging response) genes MLH1, MSH2, MSH6, and PMS2.
Dr. Saltz: We have not adequately parsed the differences between actionable and beneficial alterations. There is a difference between theoretical actionability and beneficial actionability. The concordance between these two spheres is an issue that we are wrestling with as an oncology community.
Dr. Vasan: For his metastatic melanoma, the patient was started on dabrafenib and trametinib based on the somatic V600E BRAF mutation. Surveillance imaging at three months showed improvement in liver and lung lesions.
Dr. O’Reilly: What is the standard of care for BRAF-mutated metastatic melanoma?
Dr. Shoushtari: Either targeted RAF-MEK therapy or immune-activating therapy can be utilized. The standard of care for targeted therapy of BRAF-mutated metastatic melanoma is a combination of either dabrafenib and trametinib or vemurafenib and cobimetinib. His case brings up an important clinical dilemma given that BRAF inhibitors can potentiate KRAS-driven malignancies, most notably squamous cell carcinoma (21). This risk decreases when MEK inhibitors are combined with BRAF inhibitors (22). However, there are case reports of the development of other KRAS-driven malignancies on combination BRAF and MEK inhibition including pancreas cancer (23).
Dr. Saltz : Likely the benefit of treating the BRAF-driven melanoma in the liver outweighed the small risk of a new KRAS-driven malignancy or potentiating his preexisting pancreas cancer.
Dr. Abou-Alfa: How do you decide between immunotherapy and targeted therapy for these patients?
Dr. Shoushtari: BRAF and MEK dual targeted therapy for treatment of BRAF-mutated metastatic melanoma is associated with a ~70% response rate lasting 12–15 months (24,25). Immunotherapy with anti-PD1 antibodies (e.g., nivolumab) with anti-CTLA-4 antibodies (e.g., ipilimumab) for treatment of metastatic melanoma (regardless of BRAF status) is associated with a ~60% response rate lasting ~30 months (26). These therapies have not been compared directly as first line agents in patients with BRAF-mutated metastatic melanoma. Patients with BRAF-mutated bulky metastatic disease who need expedient cytoreduction may benefit more from the targeted therapies.
Dr. Vasan: The most recent surveillance imaging of the patient showed partial response in his liver lesions.
Dr. O’Reilly: This case exemplifies the diagnostic dilemma of new metastases in a patient with multiple malignancies and the utility of sound clinical judgment in deciding who may benefit from re-biopsy. How we as oncologists will deal with the plethora of genetic information in making management decisions for our patients remains an important research and clinical question.
Acknowledgements
This case was presented at the MSKCC/American University of Beirut/National Guard Hospital gastrointestinal cancer conference on April 13, 2016 and was supported by the endowment gift of Mrs. Mamdouha El-Sayed Bobst and the Bobst Foundation.
Footnote
Conflicts of Interest: A.N.S.: Research funding from Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
References
- Ralls PW, Halls J, Renner I, et al. Endoscopic retrograde cholangiopancreatography (ERCP) in pancreatic disease: a reassessment of the specificity of ductal abnormalities indifferentiating benign from malignant disease. Radiology 1980;134:347-52. [Crossref] [PubMed]
- Del Prete SA, Maurer LH, O'Donnell J, et al. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep 1984;68:1403-5. [PubMed]
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745-51. [Crossref] [PubMed]
- Klein AP. Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer 2013;13:66-74. [Crossref] [PubMed]
- Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015;17:251-64. [Crossref] [PubMed]
- Salo-Mullen EE, O'Reilly EM, Kelsen DP, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015;121:4382-8. [Crossref] [PubMed]
- Mork ME, You YN, Ying J, et al. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol 2015;33:3544-9. [Crossref] [PubMed]
- Saltz LB. Genetic Screening in All Young Patients With Colorectal Cancer? J Clin Oncol 2016;34:1560. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- O'Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246-50. [Crossref] [PubMed]
- Neoptolemos JP. ESPAC-4: A multicenter, international, open-label randomized controlled phase III Trial of Adjuvant Combination Chemotherapy of Gemcitabine (GEM) and Capecitabine (CAP) Versus Monotherapy Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma. JCO 2016;34(suppl;abstr LB A4006).
- Tempero MA, Cardin DB, Biankin A, et al. APACT: A phase 3 randomized, open-label, multicenter trial evaluating the use of adjuvant nab-paclitaxel (nab-P) plus gemcitabine (G) versus G alone in patients (pts) with surgically resected ductal pancreatic adenocarcinoma (PDA). J Clin Oncol 32:5s, 2014 (suppl; abstr TPS4162^)
- Trial Comparing Adjuvant Chemotherapy With Gemcitabine Versus mFolfirinox to Treat Resected Pancreatic Adenocarcinoma. Available online: https://clinicaltrials.gov/show/NCT01526135
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Gemcitabine Hydrochloride With or Without Erlotinib Hydrochloride Followed By the Same Chemotherapy Regimen With or Without Radiation Therapy and Capecitabine or Fluorouracil in Treating Patients With Pancreatic Cancer That Has Been Removed By Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01013649
- Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol 2010;28:3042-7. [Crossref] [PubMed]
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801-6. [Crossref] [PubMed]
- de Juan Jiménez I, García Casado Z, Palanca Suela S, et al. Novel and recurrent BRCA1/BRCA2 mutations in early onset and familial breast and ovarian cancer detected in the Program of Genetic Counseling in Cancer of Valencian Community (eastern Spain). Relationship of family phenotypes with mutation prevalence. Fam Cancer 2013;12:767-77. [Crossref] [PubMed]
- Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339:957-9. [Crossref] [PubMed]
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207-15. [Crossref] [PubMed]
- Carlos G, Anforth R, Clements A, et al. Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination With MEK Inhibitors for Metastatic Melanoma. JAMA Dermatol 2015;151:1103-9. [Crossref] [PubMed]
- Carlino MS, Kwan V, Miller DK, et al. New RAS-mutant pancreatic adenocarcinoma with combined BRAF and MEK inhibition for metastatic melanoma. J Clin Oncol 2015;33:e52-6. [Crossref] [PubMed]
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [Crossref] [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]


