Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion
Introduction
Cholangiocarcinoma (CCA) is an uncommon malignancy in the Western world with an annual incidence rate of 0.35–2.0 per 100,000 (1). Nevertheless, the incidence of CCA has been increasing in the developed countries.
As of today managing and caring for patients with CCA is a demanding and challenging task for their treating oncologists. Although radical surgery can offer a chance for cure, unfortunately around 80–90% of cases are locally advanced or metastatic at diagnosis due to the insidious onset of the disease (2). Despite surgical resection a recurrence rate of 60–80% has been observed (1). Optimal strategy on adjuvant treatment has not been well-defined (3). As for advanced disease oncological treatment is mainly restricted to palliative care with the aim of preventing progression. The standard first line chemotherapy is the combination of cisplatin and gemcitabine (4). Alternative regimens include gemcitabine with oxaliplatin or capecitabine, capecitabine with oxaliplatin or cisplatin, 5-fluorouracyl with cisplatin or oxaliplatin and monotherapy with gemcitabine, 5-fluorouracyl or capecitabine (3). No standard second-line chemotherapeutic regimen exists. Patients with unresectable CCA have a median overall survival (OS) of less than a year and a 5-year survival rate of less than 10% (1,2). Due to the dismal prognosis with conventional chemotherapy, attention has been shifting to the potential role of molecularly targeted agents in advanced CCA. Several clinical trials had been carried out, where targeted agents were applied alone or in combination with chemotherapy with only modest or no proven benefit at all (5-8). One main limitation of most trial design was subject heterogeneity with regards to the anatomical and molecular characteristics of the cholangiocellular malignancies.
Here we report a case of a patient with rapidly progressing metastatic extrahepatic cholangiocarcinoma (EHCCA) where combination of dabrafenib plus trametinib achieved a dramatic therapeutic response after failure of first line gemcitabine and cisplatin therapy. We demonstrate the decision making process of the multidisciplinary tumor board how targeted therapy was initiated based on the results of next generation sequencing (NGS).
Case presentation
A 59-year-old-woman presented with abdominal discomfort and jaundice due to biliary obstruction. Her medical history included hypertension, type 2 diabetes mellitus, hypothyroidism and atrial septal defect which required closure by catheter-technique. Computed tomography (CT) revealed a mass-forming lesion involving the pancreatic head and distal biliary tract, dilated biliary tree and several metastatic lymph nodes in the chest (mediastinal and hilar) and abdominal cavity (hepatic hilar, retroperitoneal, mesenterial). Chest imaging was inconclusive about the presence of lung metastasis, but positron emission tomography (PET) CT demonstrated lymphangitis carcinomatosa of the right lung. Brain magnetic resonance imaging (MRI) confirmed multiple cerebellar and cerebral metastases with a maximum diameter of 13 mm. At presentation highly elevated serum bilirubin level (338 µmol/L) was detected. The patient’s baseline Eastern Cooperative Oncology Group (ECOG) performance status was 1. Biliary tract decompression with stent placement in the right hepatic and common bile ducts was performed with percutaneous approach. Ultrasound-guided fine needle aspiration (FNA) was performed from the pancreatic lesion, with parallel transbronchial FNA sampling from the pathologic hilar lymph nodes. Thorough pathologic examination unequivocally confirmed poorly differentiated adenocarcinoma of the distal bile tract.
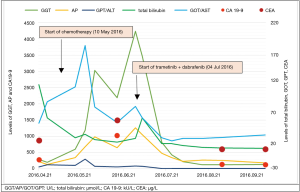
Multidisciplinary tumor board recommended whole brain radiation therapy and systemic chemotherapy with cisplatin plus gemcitabine. A total dose of 30 Gray (Gy) was given in 3 Gy fractions to the intracranial metastases. After the completion of WBRT, combination chemotherapy of cisplatin and gemcitabine had been initiated. By this time serum bilirubin level had decreased to normal. Chemotherapeutic regimen included 25 mg/m2 cisplatin and 1,000 mg/m2 gemcitabine administered on day 1 and day 8 every three weeks. During the first two cycles of chemotherapy the patient’s performance status declined to ECOG 3 while serum levels of cholestatic enzymes and tumour markers progressively increased (Figure 1). Restaging CT demonstrated obvious progression regarding the primary tumor and metastatic lymph nodes, moreover right sided pleural effusion appeared as a new lesion. Planned thoracocentesis could not be performed due to patient refusal. Brain MRI demonstrated stable status. Rapid progression of the disease and the lack of standard second-line chemotherapy in CCA orientated our therapeutic strategy to targeted agents.
NGS based tumor molecular profiling was performed on the aspiration cytological sample of the primary tumor followed by biological and clinical interpretation of these results, all of which had been performed via Oncompass Medicine Hungary Ltd. expert team. Proprietary algorithm of Oncompass Medicine was used for the molecular and clinical interpretation of these diagnostic results.
Tumor cell ratio of the studied sample was determined to be 80% by the pathologist. Deoxyribonucleic acid (DNA) was extracted from the sample by Qiamp® DNA FFPE Tissue Kit following the manufacturer’s protocol. In total, 332 amplicons of 58 genes were PCR amplified. Barcoded DNA sequencing run was performed on an Ion Torrent PGM system (318 chip) (Life Technologies, Carlsbad) following the manufacturer’s protocol.
The following four non-synonymous alterations were identified involving both onco- and tumor suppressor genes: BRAF (p.V600E) in 15%, TP53 (p.Q16fs*28) in 22%, PIK3R1 (p.M326I) in 41%, and ATM (p.R337H) in 15% of the tested DNA. Based on public and internal databases (e.g., Catalogue of Somatic Mutations in Cancer) as well as literature search PIK3R1-M326I was classified as a non-driver (9), while ATM-R337H was classified as a variant of uncertain significance (VUS) due to lack of sufficient scientific evidence being a driver versus non-driver. On the other hand BRAF V600E and TP53-p.Q16fs*28 were considered as clinically relevant driver mutations (10,11). Using ranking based algorithm of scientific evidence and clinical experience matching targeted drugs and compounds were identified as potential therapeutic options including available molecularly and clinically matching clinical trials for the patient.
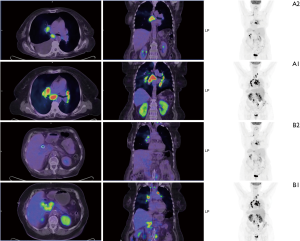
Based on the favourable results of a case report with CCA (9) and the known central nervous system (CNS) involvement of our patient (12) combination therapy with BRAF and MEK inhibitor was suggested by the multidisciplinary tumor board. Dabrafenib (Tafinlar®, Novartis) 150 mg twice a day and trametinib (Mekinist®, Novartis) 2 mg daily was initiated. Follow-up visits were scheduled every two weeks. Dramatic improvement in the patient’s general condition and significant decrease of the cholestatic enzyme levels were observed on the first control and this tendency has been continuously seen (Figure 1). To monitor the potential side effects, the patient is under monthly dermatological, ophthalmological and cardiological control beyond the regular physical examination, routine laboratory tests and ECG, which are done at the oncological visits every two weeks. Except for grade 1 nausea, so far no adverse events of BRAF or MEK inhibitor had been observed. First restaging CT was performed eight weeks after the initiation of the combined targeted therapy. Chest and abdominal imaging demonstrated almost complete resolution of the pleural effusion, disappearance of the thoracic metastatic lymph nodes, spectacular decrease in size of the abdominal lymph nodes as well as at the primary tumor. Despite these dramatic response, a new hepatic lesion of 67 mm × 40 mm appeared in the left lobe. The radiologist assessed the hepatic lesion as a new metastasis and reported progressive disease according to the definition of the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (13). Brain MRI revealed stable disease. By that time tumour markers (CA 19.9, CEA) and biliary enzymes markedly decreased (Figure 1). Since the clinical and radiological data were contradictory, CT-guided biopsy was performed from the newly developed hepatic mass. Pathologic examination revealed an inflammatory lesion with fibrous tissue, while tumor cells were not detected in the sample. Due to the benign nature of the liver mass and the lack of systemic inflammatory signs therapeutic response was regarded as partial response according to RECIST version 1.1 and combined treatment of dabrafenib plus trametinib was continued. At 12 weeks second restaging was performed with PET CT demonstrating further tumor regression and complete radiologic response regarding multiple cerebral and cerebellar metastases (Figure 2). The inflammatory hepatic lesion showed regression too.
As a result of the continuous and dramatic response and the lack of any significant side effects dabrafenib and trametinib therapy is being continued with an unchanged dose, for 6 months at the time of finalization of the manuscript.
Discussion
This is the first reported case on successfully treating an EHCCA with dabrafenib and trametinib combination in second line based on NGS results using aspiration cytological sample.
CCA is a pathogenetically complex disease originating from the epithelial lining of the intrahepatic (IH) or extrahepatic (EH) biliary ducts (2). Although subtypes of CCA can show some overlap in pathogenicity, they are diverse in their anatomical location, clinical behaviour and molecular profile (5-8,14,15). Since the prognosis of CCA remains poor with traditional chemotherapy, in the era of personalized medicine several research teams have been working on the molecular and genetic characterization of CCA to identify molecular targets (5-8,14). Landscape of signalling pathways offers multiple therapeutic targets including growth factor receptors (GFRs) like epidermal (EGFR), vascular (VEGFR), human epidermal growth factor receptor 2 (HER2), the ligand of VEGFR (VEGF) and their downstreaming signal molecules of RAS-RAF-MEK-ERK and PI3K-ACT-mTOR (5-8,14). Target-specific monoclonal antibodies and tyrosine kinase inhibitors are able to block selectively the over-expressed or over-activated signal molecules potentiating the growth and invasion of CCA cells. Based on the success of targeted therapy in colorectal, breast, lung cancer and melanoma, targeted treatment could be effective in advanced CCA, as well. However as frequency and distribution of the potentially actionable targets are exceptionally variable in CCA, comprehensive genomic testing seems to be a reasonable option before initiation of a targeted treatment (14).
In our case report BRAF V600E mutation was identified in the primary tumor via NGS. The over-activated BRAF, as an intracellular kinase is driving the continuous activation of the mitogen-activated protein kinase (MAPK) pathway that may contribute to the pathogenesis of several malignancies including melanoma (~50%) (16), hairy cell leukaemia (100%) (12,17) or non-small cell lung cancer (2–4%) (18). BRAF V600E is the most common BRAF mutation leading to a substitution of valine for a glutamate at codon 600. Small-molecule inhibitors of BRAF V600 kinase (vemurafenib, dabrafenib) significantly improved survival compared with chemotherapy in metastatic melanoma with BRAF V600 mutation, though resistance usually occcurs after a median 6–7 months and second malignancies can appear (19,20). BRAF inhibitors can provoke the reactivation of MAPK pathway downstream of BRAF kinase in a ras-dependent manner (21). Paradoxical activation of the MAPK pathway occurs not only in BRAF-mutant melanoma cells, but in the BRAF wild-type and ras-mutant normal cells driving the appearance of secondary cancers like hyperproliferative cutaneous lesions (21), primary melanoma (22) or leukaemia (23). Dual inhibition of BRAF and MEK delaying the emergence of resistance and increases survival in patients with metastatic melanoma, as MEK inhibitor (trametinib) suppresses the acquired reactivation of the MAPK pathway (17). Not only the toxic effects were much better tolerated in combination treatment but the incidence of cutaneous squamous-cell carcinoma was lower in the dabrafenib-trametinib arm compared to dabrafenib monotherapy (21,24). Based on the available data, dabrafenib plus trametinib became the first targeted combination therapy approved by the Food and Drug Administration (FDA) in metastatic BRAF V600E mutated melanoma. A few promising data are reported on the use of BRAF plus MEK inhibitors in non-melanoma cancers harboring BRAF V600E mutations (12,24). A phase 2, multicentric, non-randomised trial of dabrafenib plus trametinib demonstrated significant antitumour activity and manageable safety profile on 57 patients with BRAF V600E mutated non-small cell lung cancer (12). A study on 43 patients with metastatic colorectal cancer with BRAF V600 mutation dabrafenib plus trametinib treatment proved to be active in a subset of patients, however the degree of response was less than in BRAF-mutant melanoma treated with dabrafenib alone (24).
In CCA the frequency of BRAF V600E has been reported to range 0–12% in EH and 0–22% in IH subtypes (5-8,14). Although BRAF V600 can serve as a potential molecular target in CCA, until now there was no evidence of benefit using BRAF inhibitor therapy. Early clinical trials are still ongoing and only limited number of case reports exist. Vemurafenib presented only modest antitumor activity in a case of advanced CCA with BRAF V600 mutation (25,26). According to our knowledge, only one case report is available on the successful administration of dabrafenib plus trametinib in metastatic CCA with BRAF V600E mutation (27). That particular case is quite similar to the case presented here, still there are considerable differences. Our case proves that BRAF plus MEK inhibitor therapy is safe to use and effective as a second line treatment in chemotherapy refractor CCA while the case presented by Loaiza-Bonilla and colleagues reflects a successful first line treatment. In addition their case is an IHCCA, while our patient has an EHCCA. Furthermore, our case had CNS involvement that showed complete radiologic regression by week 12 post dabrafenib and trametinib therapy. We would like to draw the attention to the new hepatic lesion mimicking progression, later proved to be an inflammatory lesion that was attributed to the underlying biliary obstruction. However contribution of BRAF/MEK inhibitor therapy to the fibrotic, inflammatory lesion could not be ruled out (28).
It is noteworthy to mention that in our case the molecular characterization of the primary tumor was performed effectively from FNA cytology sample.
Conclusions
This case presentation proves that combined BRAF and MEK inhibitor therapy is feasible in cholangiocarcinoma harboring BRAF mutation and potentially could be as effective as in other malignancies with BRAF mutation. In the era of targeted agents, systemic therapeutic approaches in advanced CCA are shifting form the marginally effective traditional chemotherapy to personalized medicine. Considering the molecular heterogeneity of CCA, comprehensive molecular and genetic profiling can be advisable to select a potential target. As CCA is relatively uncommon, frequencies of the individual druggable mutations are even rarer, performing clinical trials on this patient group is very difficult. Therefore case presentations have a real value to demonstrate the efficacy and rationality of targeted therapies orientated by molecular profiling in advanced CCA.
Acknowledgements
Zsolt Lengyel (MD), Kornélia Kajáry (MD) and István Kozma (MD), Pozitron-Diagnostic Center, Budapest, Hungary, provided PET CT images.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am Soc Clin Oncol Educ Book 2016;35:e194-203. [Crossref] [PubMed]
- Brandi G, Venturi M, Pantaleo MA, et al. Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig Liver Dis 2016;48:231-41. [Crossref] [PubMed]
- Benson AB 3rd, Abrams TA, Alberts SR, et al. NCCN Practice Guidelinesin Oncology (NCCN Guidelines(R)) - Hepatobiliary Cancers 2016. Cited 2016 06/27. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 2016;7:46750-67. [PubMed]
- Oyasiji T, Zhang J, Kuvshinoff B, et al. Molecular Targets in Biliary Carcinogenesis and Implications for Therapy. Oncologist 2015;20:742-51. [Crossref] [PubMed]
- Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol 2016;22:1335-47. [Crossref] [PubMed]
- Brandi G, Farioli A, Astolfi A, et al. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget 2015;6:14744-53. [Crossref] [PubMed]
- Almind K, Delahaye L, Hansen T, et al. Characterization of the Met326Ile variant of phosphatidylinositol 3-kinase p85alpha. Proc Natl Acad Sci U S A 2002;99:2124-8. [Crossref] [PubMed]
- Saridaki Z, Tzardi M, Sfakianaki M, et al. BRAFV600E mutation analysis in patients with metastatic colorectal cancer (mCRC) in daily clinical practice: correlations with clinical characteristics, and its impact on patients' outcome. PLoS One 2013;8:e84604. [Crossref] [PubMed]
- Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell 1992;70:523-6. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 2016;7:797-803. [Crossref] [PubMed]
- Cohen PR, Bedikian AY, Kim KB. Appearance of New Vemurafenib-associated Melanocytic Nevi on Normal-appearing Skin: Case Series and a Review of Changing or New Pigmented Lesions in Patients with Metastatic Malignant Melanoma After Initiating Treatment with Vemurafenib. J Clin Aesthet Dermatol 2013;6:27-37. [PubMed]
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med 2012;10:85. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305-15. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207-15. [Crossref] [PubMed]
- Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol 2015;16:1389-98. [Crossref] [PubMed]
- Callahan MK, Rampal R, Harding JJ, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med 2012;367:2316-21. [Crossref] [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023-31. [Crossref] [PubMed]
- Silkin SV, Startsev SS, Krasnova ME, et al. Complete Clinical Response of BRAF-Mutated Cholangiocarcinoma to Vemurafenib, Panitumumab, and Irinotecan. J Gastrointest Cancer 2016;47:502-5. [Crossref] [PubMed]
- Pattanaprichakul P, Tetzlaff MT, Lapolla WJ, et al. Sweet syndrome following vemurafenib therapy for recurrent cholangiocarcinoma. J Cutan Pathol 2014;41:326-8. [Crossref] [PubMed]
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 2014;8:479. [Crossref] [PubMed]
- Marsico JG, Rodriguez R, Müller J, et al. Vemurafenib-related sterile scrotal abscess in a patient with BRAFV600K-mutant advanced melanoma mimicking distant metastasis. J Cancer Res Ther 2015;11:647. [Crossref] [PubMed]