Targeted therapies in colorectal cancer: surgical considerations
Background
Colorectal cancer is the 4th deadliest cancer worldwide (1). The liver, followed by the lung, is the most common site of distant metastatic disease. Indeed, for nearly 1/3 of patients with metastatic colorectal cancer (mCRC), the liver is the only affected visceral organ (2). Approximately 15-25% of patients have synchronous liver metastasis at the time of their initial colorectal cancer diagnosis and 10-25% of patients develop metachronous liver metastasis sometime after curative resection of the primary lesion (3-5). Unfortunately, even when metastatic disease remains limited to the liver, the majority of these metastases are unresectable and the reported rates of successful resection have ranged between 20-30% (6,7). These rates of successful curative resection are relevant mostly from a historical perspective and likely underestimate current surgical practice given the recent advances in systemic therapies. Since the selection and timing of therapeutic agents in patients with mCRC is complex, especially in relation to surgical intervention, each component of the multimodality management of patients with mCRC must be carefully planned to provide the best overall outcomes.
Evolution of systemic chemotherapy for metastatic colorectal cancer
Before surgical advances allowed safe resection of colorectal liver metastases (CRLM), patients were treated primarily with systemic therapies. In fact, over two decades have passed since bolus 5-fluorouracil (5-FU) was the standard of care for patients with mCRC (8-10). Variations in the administration of 5-FU and combinations with agents to modulate its activity [levamisole and leucovorin (LV)] produced incremental improvements in patient outcomes; however, median overall survival (OS) largely remained near 12 months (11-14). A major advance in systemic therapies for mCRC was reported in 2000 when two phase III trials showed that the addition of irinotecan (CPT-11), a DNA topoisomerase I inhibitor, to 5-FU/LV significantly increased overall response rates (ORR), progression-free survival (PFS), and OS (15-17). In the report by Saltz et al., weekly treatment consisted of irinotecan (125 mg/m2), bolus 5-FU (500 mg/m2), and LV (20 mg/m2) (IFL) (15). In the 2nd trial, Douillard et al., observed improved outcomes using bi-weekly FOLFIRI (irinotecan, 180 mg/m2; LV, 200 mg/m2; and bolus 5-FU, 400 mg/m2 followed by 22 h infusional 5-FU, 600 mg/m2) (16). These positive studies led to the acceptance of the combination of irinotecan with 5-FU/LV for first-line therapy of mCRC.
During the same period of time that improvements with irinotecan were observed, oxaliplatin, a platinum-based agent that blocks DNA replication, was also tested in combination with 5-FU/LV (FOLFOX) for patients with mCRC (18). In a phase III study reported by de Gramont et al., patients who were administered FOLFOX4 (LV, 200 mg/m2; 5-FU, 400 mg/m2 bolus followed by 22 h infusion of 600 mg/m2; and oxaliplatin, 85 mg/m2) had improved ORR and prolonged PFS, although increases in OS did not reach statistical significance (19). This study led to the acceptance of FOLFOX as another option for first-line treatment of patients with mCRC.
More recently, the combination of oxaliplatin and irinotecan has also been explored. In a randomized phase III study by Falcone et al., patients received either 48-h infusional 5-FU (3,200 mg/m2), LV (200 mg/m2), oxaliplatin (85 mg/m2), and irinotecan (165 mg/m2) (FOLFOXIRI) vs. FOLFIRI (20). The FOLFOXIRI regimen was associated with significantly increased ORR (66% vs. 41%, respectively), PFS (9.8 vs. 6.9 months, respectively), and OS (median, 22.6 vs. 16.7 months, respectively). Even though FOLFOXIRI was also associated with higher levels of Grade 2/3 toxicities, the FOLFOXIRI regimen has been accepted as another first-line therapeutic option for patients with mCRC.
Emergence of targeted therapies for metastatic colorectal cancer
Although outcomes have improved with advances in systemic chemotherapy for mCRC, potent small molecules and antibodies targeting specific proteins have also been developed over the past decade and have further improved the efficacy of standard chemotherapy regimens. The first of these aptly named “targeted agents” to show benefit as first-line therapy for patients with mCRC was bevacizumab, a recombinant humanized monoclonal IgG1 antibody targeting vascular endothelial growth factor (VEGF). Hurwitz et al. showed that patients with mCRC who received bevacizumab + IFL had significantly better ORR (44.8% vs. 34.8%, respectively), PFS (10.6 vs. 6.2 months, respectively), and OS (median, 20.3 vs. 15.6 months, respectively) compared to IFL alone (21). By virtue of its mechanism of action as an anti-angiogenesis agent, bevacizumab must be used with caution in both medical and surgical patients because of known adverse events including gastrointestinal perforation, hemorrhage, and impaired wound healing (22,23).
The second well-established molecular target in mCRC is epidermal growth factor receptor (EGFR), which is overexpressed in nearly 85% of colorectal cancers (24,25). Cetuximab, a chimeric IgG1 monoclonal antibody directed against the external surface of EGFR, was first evaluated in combination with chemotherapy in patients who were refractory to irinotecan and also as a single agent in patients intolerant to standard chemotherapy (26-29). These randomized, phase II and phase III trials showed improved PFS without differences in OS (29). More recently, Van Cutsem et al., demonstrated an OS benefit with cetuximab when the cohort was limited to patients with wild-type KRAS in their cancers (30). A 2nd EGFR-targeted antibody, panitumumab is a fully humanized IgG2 monoclonal antibody that was initially approved by the FDA as a third-line treatment for mCRC in 2007 (31). The PRIME trial utilized a combination of panitumumab + FOLFOX4 in patients with wild-type KRAS that revealed improved PFS but a non-significant increase in OS compared to FOLFOX4 alone. Currently, panitumumab is FDA-approved for use in patients with refractory mCRC (32). A summary of the major trials demonstrating benefit with standard and targeted agents in mCRC is listed in Table 1.
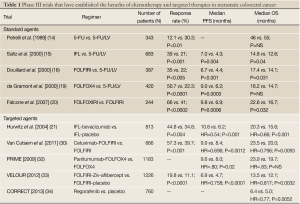
Full Table
Paradigm shift in surgical resection of colorectal liver metastases
Although contemporary therapeutic regimens have increased the longevity of patients with CRLM, the only option for cure remains complete resection of the metastatic disease. Fortunately, the improvements in medical therapies for mCRC have been concomitant with refinements in surgical and critical care techniques and technologies. Routinely, patients who undergo hepatic resection for CRLM now have 5-year survival rates nearing 40% or higher (35-38). In the past only a fraction of the one-quarter of patients with mCRC limited to the liver were considered for curative surgical options. Much has changed with the advent of more powerful chemotherapy regimens and effective targeted agents. The response rates have increased and patients who in the past would have been considered never resectable are now approached with treatment plans with intent for cure. Since surgical resection represents the only curative option for CRLM, the definition of resectability, the timing of hepatic metastasectomy, the role of maximizing treatment response, and the effect of chemotherapy and targeted agents on surgical outcomes are all key issues that must be addressed.
Consideration of surgery for CRLM mandates a clear and reproducible definition of resectable liver disease. Although the relative criteria for resectability may vary among institutions, the absolute criteria are generally the same. First, the designation that CRLM is resectable must indicate that complete microscopic negative margin resection (i.e., R0) can be achieved with adequate future liver remnant (FLR). Second, absolute contraindications to hepatic resection include current or expected hepatic failure, the presence of unresectable extrahepatic disease, and medical co-morbidities precluding safe surgical intervention. Prior randomized trials have used the following criteria to define unresectable disease: >4 metastases, tumor size >5 cm, bilobar involvement, and involvement of major vascular structures (39,40). However, these outdated criteria have been largely replaced by the goal for R0 resection with appropriate FLR, generally more than 20% in normal livers and >30% in livers with impaired function (41-43). The emphasis on R0 resection is important, because positive resection margins predict an unfavorable prognosis (37). Although a 1-cm margin was traditionally defined as an adequate margin, more recent studies suggest that any negative margin is acceptable (35,44).
The timing of hepatic metastasectomy in patients presenting with primary colorectal cancers and synchronous CRLM is another dilemma. Simultaneous colorectal resection and hepatic metastasectomy may be considered to limit the risks of morbidity and mortality with the 2nd operative procedure. De Haas et al., reported fewer overall complications with simultaneous colorectal resection and liver metastasectomy (11% vs. 24%, respectively); but mortality rates were similar when compared to staged resections (45). Other studies have reported similar rates for both morbidity and mortality with simultaneous resection compared to staged resections (46-48). Despite these results, some centers still support a staged resection, with initial colorectal resection followed by future interval/delayed hepatic resection (35,49,50). The management of metachronous CRLM disease is generally straightforward and involves initial colorectal resection and later resection of CRLM.
Treatment algorithms for patients with CRLM have evolved because of improved response rates with the addition of targeted agents to treatment regimens. Multiple trials have been shown to significantly increase response rates when adding bevacizumab or cetuximab to irinotecan or oxaliplatin backbone regimens (51-54). For example, cetuximab was evaluated in the phase II multi-center CELIM trial. Patients with unresectable CRLM were randomized to receive cetuximab with either FOLFOX6 or FOLFIRI (52). The ORR was 68% in the FOLFOX6 arm and 57% in the FOLFIRI arm (52). R0 liver resection was subsequently performed in 20 of 53 (38%) patients in the cetuximab/FOLFOX6 group and in 16 of 53 (30%) patients in the cetuximab/FOLFIRI group. The increases in ORRs have ranged between 10-30% with corresponding increased rates of hepatic resection of 5-20% when cetuximab was combined with chemotherapy across most studies (29,52,55). Improvements in ORRs and subsequent rates of surgical resection have also been observed with bevacizumab. In the First Bevacizumab Expanded Access Trial (First BEAT), bevacizumab was added to the investigator’s choice of fluoropyrimidine-based chemotherapy for patients with CRLM (54). Of 1,914 patients, 225 were able to undergo surgery with curative intent (11.8%). Resection rates were higher in patients receiving oxaliplatin-based chemotherapy (16.1%) than in those receiving irinotecan-based chemotherapy (9.7%). Finally, Falcone et al. reported a 66% ORR with FOLFOXIRI alone, whereas response rates with single backbone chemotherapy regimens in most trials were much lower and ORRs have generally increased with the addition of bevacizumab or cetuximab (20,21,51).
Despite great improvements in response rates and resectability with standard and targeted agents, chemotherapy has the potential for liver damage and toxic side-effects that can affect surgical outcomes. Significant decreases in liver function have been described with 5-FU, oxaliplatin, and irinotecan and can contribute to increased perioperative morbidity (43,56). Steatohepatitis, the accumulation of lipids in hepatocytes leading to inflammation and fibrosis, has been associated with irinotecan, while oxaliplatin can cause sinusoidal dilation, perisinusoidal fibrosis, and occlusion of venules (56-58). To offset the effects of chemotherapy-associated liver injury, a delay period from the last dose of chemotherapy to resection of CRLM is required. The National Comprehensive Cancer Network (NCCN) recommends waiting one month from the last dose of chemotherapy to surgery (59). A time interval of at least 4-6 weeks after the last dose of chemotherapy is also supported by major trials (52,54,60). Interestingly, while sinusoidal injury resulting in the “blue liver” syndrome has been attributed to oxaliplatin, bevacizumab may have a protective effect by decreasing the severity of sinusoidal obstruction and damage (61). Bevacizumab has also been associated with non-liver adverse effects such as impaired wound healing and increased risk of intestinal perforation due to its anti-angiogenesis properties (23,62,63). For surgical patients who have received bevacizumab, the NCCN recommends wait-times of approximately 4-6 weeks after the last bevacizumab dose before surgery (59). For the anti-EGFR agents cetuximab and panitumumab, no specific liver toxicity, wound healing, or other adverse effect which impact surgical care has been reported; hence, the necessary wait period is similar to that for non-targeted agents (64,65).
Preoperative treatment strategies
Patients with CRLM may present in a number of different manners. Common presentations include: (I) unresectable disease; (II) borderline resectable disease; and (III) resectable disease. The role of systemic agents and targeted therapies may be different in each of these conditions (see Figure 1). For patients with CRLM who are initially declared unresectable, therapies may be given to optimize shrinkage of the tumor to convert initially unresectable to resectable disease. This so called “conversion” therapy may be similar to standard chemotherapy regimens when patients are considered never resectable. For patients undergoing treatment for initially unresectable CRLM, the close involvement of the surgical team is essential. Patients should be reevaluated for possible surgical resection after two months of therapy and every two months thereafter if treatment is continued.
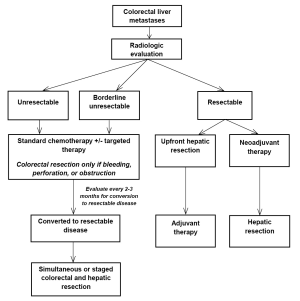
Neoadjuvant therapy is the administration of therapy to patients who have CRLM that is considered resectable at time of diagnosis. Advantages to neoadjuvant chemotherapy include decreasing the size of the CRLM to allow less extensive liver resection and greater likelihood of margin negative resection and evaluating disease biology during treatment. Furthermore, chemosensitivity and responsiveness can be determined by evaluating treatment response. Perioperative therapy (i.e., preoperative and postoperative) with standard regimens was tested in the EORTC 40983 trial, which evaluated the role of chemotherapy in patients with resectable CRLM. Increased PFS was observed in the perioperative FOLFOX4 arm compared to surgery alone (66), however, follow-up survival analysis did not demonstrate significant differences in OS between the two treatment arms (67).
Adjuvant chemotherapy and targeted agents
After resection of liver metastases, up to 70% of patients may develop recurrence of disease either in the liver or in extra-hepatic locations, thus providing rationale for postoperative or adjuvant chemotherapy (68). However, data for systemic therapies in this setting is severely lacking. If data from patients with stage III disease were extrapolated to stage IV patients, then chemotherapy regimens would be recommended since recurrence was lower and OS was higher with adjuvant chemotherapy. However, neither bevacizumab nor cetuximab in the adjuvant setting provided survival benefits when combined with chemotherapy in stage III trials (69,70). Regardless, it may not be reasonable to compare complete resection of disease in stage III patients who have locoregional disease with stage IV patients who have distant metastatic disease. Currently, no Level 1 recommendation based on a randomized trial can be made regarding adjuvant targeted therapy after resection of CRLM. Nevertheless, most patients will receive some form of adjuvant therapy given the improved outcomes with standard and targeted therapies in patients with mCRC.
Management of the primary tumor
The management of the primary tumor is a topic of controversy in patients with unresectable mCRC. The current strategy is to leave the primary cancer in place unless there are complications that include bleeding, obstruction, or perforation. This strategy is based upon the observation that patients receiving chemotherapy or targeted agents do not have increased rates of complications or emergent resections (NSABP C-10) (71). However, a recent retrospective analysis suggested a potential survival benefit with resection of the primary tumor when mCRC was unresectable (72). More work is needed to clarify the most appropriate management of the primary tumor in patients with unresectable mCRC.
The future is now: novel targeted agents
Ziv-aflibercept and regorafenib are two newly approved targeted agents for mCRC. Ziv-aflibercept is a soluble recombinant protein that acts as a “trap” for multiple angiogenic factors (73). This protein interferes with angiogenesis by binding to VEGF-A, VEGF-B, and placental growth factor (PlGF), thus “trapping” these growth factors and preventing binding to and activation of VEGF receptors, thereby interfering with angiogenesis. In the phase III randomized, double-blind, multi-national VELOUR trial, patients with mCRC previously treated with oxaliplatin were randomized to receive ziv-aflibercept or placebo every two weeks in combination with FOLFIRI (33) with the primary endpoint of OS. At a median follow-up time of 22.3 months, patients receiving ziv-aflibercept had significant increases in both OS (median, 13.5 vs. 12.1 mos, respectively) and ORR (19.8% vs. 11.1%, respectively) when compared to placebo. Thus, ziv-aflibercept is now FDA approved for second-line use in combination with FOLFIRI or irinotecan in patients with disease progression on oxaliplatin. There are no studies in surgical patients as of yet.
Another oral agent, regorafenib, has also been investigated in the treatment of mCRC. Regorafenib inhibits multiple tyrosine kinases and possesses anti-angiogenic properties, specifically targeting VEGFR1-3, the angiopoietin receptor TIE2, RAF, PDGFR, fibroblast growth factor receptor (FGFR), as well as KIT and RET (74,75). In the multi-national phase III CORRECT trial, patients with mCRC who had progressed on standard therapy were randomized to regorafenib or best supportive therapy with a primary endpoint of OS. Patients who received regorafenib had improved OS (median, 6.4 vs. 5 mos, respectively) (34). Therefore, regorafenib is now indicated as a single agent in patients with mCRC refractory to chemotherapy. Currently there is no data in surgical patients; therefore, retrospective reports and prospective trials will help determine the role and safety of these agents in surgical patients with CRLM.
Summary
Great advances have been made in the management of patients with mCRC in the past three decades. Without treatment, patients with CRLM had a life expectancy of 4.5-12 months (76,77). The prognosis of patients with metastatic colorectal cancer of the liver has improved significantly over the past decade. Surgical resection of CRLM is still considered the only curative option and advances in surgical techniques and technology have increased the rates of patients with CRLM who may undergo hepatic resection. However, the management of CRLM mandates a multi-disciplinary effort because of the complexity of liver surgery and the tremendous advances in targeted therapies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 1986;150:195-203. [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [PubMed]
- Jatzko G, Wette V, Müller M, et al. Simultaneous resection of colorectal carcinoma and synchronous liver metastases in a district hospital. Int J Colorectal Dis 1991;6:111-4. [PubMed]
- Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 2003;12:165-92. [PubMed]
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [PubMed]
- Nordlinger B, Van Cutsem E, Rougier P, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 2007;43:2037-45. [PubMed]
- Cunningham D, Findlay M. The chemotherapy of colon-cancer can no longer be ignored. Eur J Cancer 1993;29A:2077-9. [PubMed]
- Leichman CG, Fleming TR, Muggia FM, et al. Phase II study of fluorouracil and its modulation in advanced colorectal cancer: a Southwest Oncology Group study. J Clin Oncol 1995;13:1303-11. [PubMed]
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J Clin Oncol 1992;10:904-11. [PubMed]
- Lokich JJ, Ahlgren JD, Gullo JJ, et al. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a Mid-Atlantic Oncology Program Study. J Clin Oncol 1989;7:425-32. [PubMed]
- Laufman LR, Krzeczowski KA, Roach R, et al. Leucovorin plus 5-fluorouracil: an effective treatment for metastatic colon cancer. J Clin Oncol 1987;5:1394-400. [PubMed]
- Davis T, Borden E, Wolberg W, et al. Levamisole and 5-fluorouracil in metastatic colorectal carcinoma. Proc Am Soc Clin Oncol 1982;1:102.
- Petrelli N, Herrera L, Rustum Y, et al. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol 1987;5:1559-65. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-7. [PubMed]
- Armand JP, Ducreux M, Mahjoubi M, et al. CPT-11 (Irinotecan) in the treatment of colorectal cancer. Eur J Cancer 1995;31A:1283-7. [PubMed]
- Raymond E, Faivre S, Woynarowski JM, et al. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol 1998;25:4-12. [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [PubMed]
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007;14:1860-9. [PubMed]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 2008;26:3523-9. [PubMed]
- Hemming AW, Davis NL, Kluftinger A, et al. Prognostic markers of colorectal cancer: An evaluation of DNA content, epidermal growth factor receptor, and Ki-67. J Surg Oncol 1992;51:147-52. [PubMed]
- Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer 2001;92:1331-46. [PubMed]
- Jonker DJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Lenz HJ, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol 2006;24:4914-21. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [PubMed]
- Wagner JS, Adson MA, Van Heerden JA, et al. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 1984;199:502-8. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [PubMed]
- Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg 2005;242:540-4; discussion 544-7. [PubMed]
- Giacchetti S, Itzhaki M, Gruia G, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 1999;10:663-9. [PubMed]
- Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol 2011;22:2042-8. [PubMed]
- Ychou M, Viret F, Kramar A, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol 2008;62:195-201. [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [PubMed]
- Ferrero A, Viganò L, Polastri R, et al. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg 2007;31:1643-51. [PubMed]
- Figueras J, Burdio F, Ramos E, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol 2007;18:1190-5. [PubMed]
- de Haas RJ, Adam R, Wicherts DA, et al. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg 2010;97:1279-89. [PubMed]
- Chua HK, Sondenaa K, Tsiotos GG, et al. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum 2004;47:1310-6. [PubMed]
- Capussotti L, Ferrero A, Viganò L, et al. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol 2007;14:195-201. [PubMed]
- Vogt P, Raab R, Ringe B, et al. Resection of synchronous liver metastases from colorectal cancer. World J Surg 1991;15:62-7. [PubMed]
- Scheele J. Hepatectomy for colorectal metastases. Br J Surg 1993;80:274-6. [PubMed]
- Lambert LA, Colacchio TA, Barth RJ Jr. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg 2000;135:473-9. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47. [PubMed]
- Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer 2010;103:1542-7. [PubMed]
- Okines A, Puerto OD, Cunningham D, et al. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer 2009;101:1033-8. [PubMed]
- Ye LC, Liu TS, Ren L, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 2013;31:1931-8. [PubMed]
- Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 2005;200:845-53. [PubMed]
- Choti MA. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Ann Surg Oncol 2009;16:2391-4. [PubMed]
- Skof E, Rebersek M, Hlebanja Z, et al. Capecitabine plus Irinotecan (XELIRI regimen) compared to 5-FU/LV plus Irinotecan (FOLFIRI regimen) as neoadjuvant treatment for patients with unresectable liver-only metastases of metastatic colorectal cancer: a randomised prospective phase II trial. BMC Cancer 2009;9:120. [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [PubMed]
- Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010;17:2870-6. [PubMed]
- Klinger M, Eipeldauer S, Hacker S, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol 2009;35:515-20. [PubMed]
- Hochster HS. Bevacizumab in combination with chemotherapy: first-line treatment of patients with metastatic colorectal cancer. Semin Oncol 2006;33:S8-14. [PubMed]
- Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007;14:1860-9. [PubMed]
- Parikh AA, Ellis LM. Targeted therapies and surgical issues in gastrointestinal cancers. Target Oncol 2008;3:119-25.
- Yau T, Chan P, Ching Chan Y, et al. Review article: current management of metastatic colorectal cancer - the evolving impact of targeted drug therapies. Aliment Pharmacol Ther 2008;27:997-1005. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. EORTC liver metastases intergroup randomized phase III study 40983: long-term survival results. [abstract]. J Clin Oncol 2012;30 Suppl 15:3508.
- Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol 2010;28:2300-9. [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controled trial. Lancet Oncol 2012;13:1225-33. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA 2012;307:1383-93. [PubMed]
- McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol 2012;30:3223-8. [PubMed]
- Faron M, Bourredjem A, Pignon JP, et al. Impact on survival of primary tumor resection in patients with colorectal cancer and unresectable metastasis: Pooled analysis of individual patients’ data from four randomized trials. J Clin Oncol 2012;30 Suppl 15:abstract 3507.
- Mitchell EP. Targeted therapy for metastatic colorectal cancer: role of aflibercept. Clin Colorectal Cancer 2013;12:73-85. [PubMed]
- Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer 2012;11:1-13. [PubMed]
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658-67. [PubMed]
- Baden H, Andersen B. Survival of patients with untreated liver metastases from colorectal cancer. Scand J Gastroenterol 1975;10:221-3. [PubMed]
- Bengtsson G, Carlsson G, Hafström L, et al. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg 1981;141:586-9. [PubMed]


