Integrating anti-EGFR therapies in metastatic colorectal cancer
Introduction
Colorectal cancer (CRC) is the 2nd leading cause of cancer-related death in the Western population and results in approximately 50,000 deaths annually in the United States (1). The incidence and mortality has decreased from 1999-2006, which is attributed to improvements in surgical and adjuvant therapy as well as increasing use of screening methods leading to earlier detection. About 20% of patients present with metastatic colorectal cancer (mCRC) and untreated, this group has a median overall survival (OS) of 7 months (2) and with therapy, a 5-year survival rate of 10% (3). The most common sites of metastasis are liver, lymph nodes, lung, and peritoneum.
Recent advances in the understanding of tumor biology and genetics has paved the way for targeted therapies and led to improvements in the efficacy of cytotoxic regimens. 5-fluorouracil (FU) has been available for use in CRC for over 60 years and eight additional agents have been approved since 1996, five of which are targeted therapies.
Epidermal growth factor receptor-the target
The epidermal growth factor receptor (EGFR) is a 170 kDa receptor tyrosine kinase, and a member of the human epidermal growth factor receptor (HER) or ErbB family. EGFR is also known as the type 1 receptor tyrosine kinase or ErbB1/HER1. The other members of the family include ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4) (4).
EGF is a potent epithelial mitogen in the gastrointestinal tract and can stimulate epithelial proliferation in the neonatal intestine. Furthermore, it can enhance the growth of primary colon epithelial cell cultures (4). Most epithelial cancers express EGFR and this growth factor receptor was the first to be proposed as a target for cancer therapy (5). The first anti-EGFR drugs were developed in 1980s and it took over 20 years for the first one to become commercially available.
The EGF receptor is composed of an extracellular ligand-binding domain, a transmembrane segment and an intracellular tyrosine kinase domain. Upon the binding of a ligand such as EGF or TGF-alpha, the EGFR forms homo- or heterodimers with the other members of the ErbB family resulting in autophosphorylation of the intracellular domain and activation of the downstream signaling pathways, which includes the MAPK pathway, the PI3K/Akt pathway and the Jak2/Stat3 pathway. This can signal cancer cell proliferation, inhibition of apoptosis, activation of invasion and stimulate tumor-induced neovascularization. Its overexpression or constitutive action has been shown to affect signaling cascades in carcinogenesis, most importantly the RAS/RAF/MAPK pathway (5). The RAS proteins are serine-threonine kinases that are activated downstream of EGFR. EGF, EGFR and TGF-α are expressed in 60-80% of colorectal cancers (4,6,7) and strong expression has been associated with decreased disease-free survival and overall survival (8-11).
Cetuximab and panitumumab
Mechanisms of action and drug overview
The mechanisms of action for EGFR inhibitors include the following properties: (I) Interference with cell-cycle progression with arrest in the G1 phase prior to DNA synthesis; (II) Antiangiogenic activity through downregulation of angiogenic factor secretion such as vascular endothelial growth factor (VEGF); (III) Inhibition of tumor cell invasion and metastasis by decreasing matrix metalloproteinase production and; (IV) Promoting apoptosis which enhances the effectiveness of cytotoxic therapy (12).
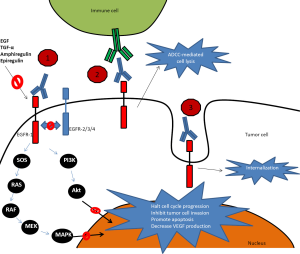
Cetuximab is a chimeric monoclonal IgG-1 antibody that was initially approved for treatment in refractory mCRC by the Food and Drug Administration (FDA) in February 2004. In July 2012 it was eventually approved in combination with 5-FU, leucovorin and irinotecan (FOLFIRI) in the first line treatment of patients with mCRC based on a phase III trial by van Cutsem et al. (13) (see further discussion in Cetuximab chapter below). Cetuximab binds to EGFR in its inactive form with higher affinity than either EGF or TGF-α and competes with other ligands by occluding the ligand-binding region and thereby ligand-induced EGFR tyrosine kinase inactivation (14). Direct inhibition of EGFR activation is considered the primary mechanism for antitumor activity for cetuximab, but other mechanisms including antibody-dependent cellular cytotoxicity (ADCC) and receptor internalization are likely to play an important role as well (see Figure 1). ADCC is dependent on interactions between the cellular FcᵧR and the monoclonal antibody, which triggers innate immunologic responses involving natural killer cells, monocytes, macrophages, activated T-lymphocytes and granulocytes. Patients with certain FcᵧR polymorphisms (FCGR2A-H131R and FCGR3A-V158F) have been shown to have higher response rates to cetuximab compared to those without this polymorphism (15). The clinical contribution of the ADCC effect is unclear and continues to be subject of investigation including methods to amplify its signal to clinical relevance, such as with lenalidomide. Receptor internalization downregulates the number of available cell surface receptors and could therefore affect EGFR activation (16).
Cetuximab is administered weekly with a loading dose of 400 mg/m2 iv over 2 hours during the first week followed by 250 mg/m2 iv over 1 hour weekly. The mean half-life is approximately 112 hours (range, 63-230 hours) (17). A small Danish study looked at giving cetuximab at 500 mg/m2 iv every other week, as pharmacokinetic studies have not revealed much differences with the two schedules, and found efficacy and safety to be similar compared to their own historical controls with weekly administration (18). The NCCN guidelines allow for using both the weekly and biweekly schedules of cetuximab as published (19).
In contrast, panitumumab is a fully humanized recombinant monoclonal IgG-2 kappa antibody which demonstrated good single-agent activity in EGFR expressing tumors in mouse models and is expected to exhibit minimal immunogenicity and therefore allow for repeated administrations without the development of antibodies (20). It was approved by the FDA as a single agent in September 2006. As ADCC is dependent upon an antibody’s subclass it is unlikely that panitumumab exerts much ADCC as it is bound to IgG-2 so its effects are mainly through blocking the receptor from binding agonists and through receptor internalization (see Figure 1). Panitumumab is approved as single agent therapy with a dosing of 6 mg/kg iv every 2 weeks and has a half-life of approximately 7.5 days (range, 3.6-10.9 days) (21).
Both cetuximab and panitumumab are cleared by receptor internalization and do not require any dose reductions for renal or hepatic impairment.
Biomarkers
The mutational status of KRAS, a Kirsten ras oncogene homolog from the ras gene family located on chromosome 12p12.1, was shown to predict responses to EGFR-targeted therapy in a study published in 2006 (22). Lievre et al. investigated 30 patients treated with cetuximab, 11 of whom had a response, for mutations in KRAS, BRAF and PIK3CA by direct sequencing as well as EGFR copy number by chromogenic in situ hybridization. They found no KRAS mutations in the 11 patients who had a response while 13 of the 19 nonresponders were found to have mutations in KRAS. None of the tumors had BRAF mutations and only 2 (7%) had exon 9 PIK3CA mutations. EGFR copy number was increased in only 3 patients but was associated with a response (P=0.004) (22). Most commonly mutations occur in codons 12, 13 or 61 in exon 2. In a large population-based study, 37% of KRAS mutations occurred within codons 12 and 13, with 6.6% occurring in codons 8, 9, 10, 15, 16, 19, 20 and 25 (23). After Lievre’s publication in 2006, multiple investigators looked at their clinical trial results with respect to KRAS mutational status and confirmed the predictive value of KRAS testing (24-31). The KRAS mutation testing became a NCCN recommendation in November 2008 (19). It should be noted that mutations in the EGFR which have been shown to predict sensitivity to tyrosine kinase inhibitors in lung cancer, are very rarely seen in colorectal cancer (32).
A search for other biomarkers have revealed mixed results with some studies showing BRAF mutations to predict lack of response (33) while others link BRAF mutations to prognosis but not response to EGFR inhibitor therapy (25). EGFR expression was initially thought to be necessary for the efficacy of EGFR inhibitor therapy. The initial trials with EGFR inhibitors were therefore restricted to patients with tumors expressing EGFR. A retrospective review and a phase II trial found responses to therapy present in patients with tumors with low or no EGFR expression and therefore suggested that expression of EGFR should not be used to select patients who would be eligible for targeted blockade (34,35).
EGFR gene copy number affects clinical outcomes in EGFR inhibitor treated patients in some but not all studies and remains controversial. A recent meta-analysis did show increased EGFR copy number to be associated with increased OS in patients receiving EGFR inhibitors as second-line therapy (HR 0.60, 95% CI, 0.47-0.75) but not as first-line therapy so this matter is still under investigation (36). However, given that increased copy number usually correlates with higher EGFR expression by immunohistochemistry, it is possible that EGFR copy number will not have a significant impact on outcome related to EGFR blockade.
A large number of patients with mCRC whose tumors show absence of KRAS mutations are non-responders. A systematic review of 8 studies published in 2008 calculated the sensitivity and specificity of KRAS testing and found KRAS mutations to have a specificity of 0.93 but a sensitivity of 0.47, demonstrating the need for further predictive biomarkers for patients with KRAS wild-type tumors (37). The EGAPP Working Group recently published recommendations for use of KRAS testing to determine likelihood of benefit with EGFR inhibitor therapy. They concluded that while sufficient evidence is available to support the predictability of KRAS mutations in codon 12 and 13, evidence is inadequate for less frequent KRAS mutations (such as in codon 61). There is also some controversy about codon 13 that will be discussed later in this review. Furthermore, they recommend against testing for BRAF, NRAS, PIK3CA and loss of expression of PTEN or AKT proteins as insufficient evidence exists to use these to guide EGFR inhibitor treatment decisions (38).
The concordance of KRAS mutational testing between the primary tumor and metastatic sites was recently reviewed in a meta-analysis looking at 19 publications with 986 paired primary and distant metastases. The study found a high concordance rate of 94.1% (95% CI, 88.3-95.0%) between primary tumor and metastatic sites while the concordance between primary tumor and lymph node metastasis was lower at 81.3% (95% CI, 69.6-97.4%) suggesting that lymph node tissue should be avoided when possible for testing (39). Either primary or metastatic tissue can be tested for KRAS per the NCCN guidelines (19).
KRAS mutational analysis in mCRC represents a negative predictive test by selecting out those patients who are unlikely to respond to anti-EGFR therapy. This represents an important step forward since in the absence of benefit, patients will avoid the potential toxicities and cost of this therapy. The absence of a mutation in KRAS will not guarantee a response and the search for positive biomarkers remains an area of intense research in mCRC.
Results of recent clinical trials (with a focus on KRAS wild type tumors)
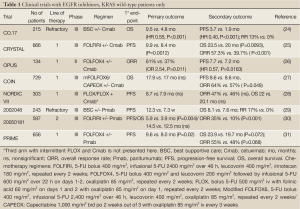
The first two trials conducted in the pre-KRAS mutational testing era showed similar efficacy for cetuximab (40) and panitumumab (41) compared to best supportive care with a distinct pattern of the progression-free survival (PFS) curves in both studies suggesting that a biomarker might explain the later separation observed. This was later identified as the presence of KRAS mutations in about 40% of patient tumor samples. After the discovery of the importance on KRAS mutational status in 2006, investigators analyzed their clinical trials selecting for KRAS status retrospectively and updated their results to confirm the importance of selecting for the absence of a mutation (Table 1).
Cetuximab
The first trial conducted with single agent cetuximab compared to best supportive care showed a significant improvement in ORR (13% vs. 0%), PFS (3.7 vs. 1.9 mo, P<0.001) and OS (9.5 vs. 4.8 mo; P<0.001) when looking at patients with KRAS wild-type tumors only. This trial did not allow for cross-over upon progression (24).
The first combination chemotherapy trial with an EGFR inhibitor was the BOND trial, published in 2004, in the pre-KRAS era. Patients who had previously progressed on irinotecan-based chemotherapy had an overall response rate (ORR) of 22%, a PFS of 4.1 months and OS of 8.6 months when treated with irinotecan and cetuximab while patients on single-agent cetuximab had an ORR of 10.8%, PFS 1.5 mo and OS 6.9 mo (42). This trial did not look at KRAS mutational status. These results suggested that EGFR inhibitors could potentially “resensitize” tumors to irinotecan after prior progression to the same agent.
The largest trial to date conducted with cetuximab is the CRYSTAL trial that explored cetuximab in combination with FOLFIRI as 1st line therapy (13). An updated analysis published in 2011 revealed that cetuximab given with FOLFIRI improved response rates (57.3% vs. 39.7%, P<0.001), median PFS (9.9 vs. 8.4 mo, P=0.0012) and median OS (23.5 vs. 20.0 mo, P=0.0093) compared to FOLFIRI alone in patients with KRAS wild-type tumors (25). The FDA approved cetuximab in conjunction with FOLFIRI as first-line therapy in July 2012 largely based on the results of this trial. Another trial, EPIC, randomized 1,298 patients who had failed prior oxaliplatin-based therapy to receive irinotecan with or without cetuximab. KRAS status was only available in 300 patients retrospectively with 36% harboring KRAS mutations. PFS was significantly longer in the KRAS wild-type group compared to the KRAS mutated group (4.0 vs. 2.8 mo, P=0.095) while RR and OS was similar. No comparison was made between patients with KRAS wild-type tumors who received or did not receive cetuximab (43).
Combining cetuximab with an oxaliplatin-based regimen has proven to have no survival benefit according to randomized phase III trials. The COIN (27) and NORDIC VII (28) trials failed to show a statistically significant survival benefit (see Table 1) while the phase II randomized OPUS trial did show an increase in median PFS (26). It should be noted that these studies used various oxaliplatin-regimens with the most modern regimen, modified FOLFOX6 (5-FU bolus 400 mg/m2, infusional 5-FU 2,400 mg/m2 over 46 h, leucovorin 400 mg/m2, oxaliplatin 85 mg/m2, repeated every 2 weeks) or CAPEOX (Capecitabine 1,000 mg/m2 bid po 2 weeks out of 3 with oxaliplatin 85 mg/m2 iv every 3 weeks) used in the COIN trial while the NORDIC trial used FLOX (bolus 5-FU 500 mg/m2 iv with folinic acid 60 mg/m2 on days 1 and 2 with oxaliplatin 85 mg/m2 on day 1, repeated every 2 weeks) and OPUS used FOLFOX4 (5-FU bolus 400 mg/m2 and leucovorin 200 mg/m2 followed by infusional 5-FU 600 mg/m2 over 22 h on days 1-2, oxaliplatin 85 mg/m2, repeated every 2 weeks). The COIN trial was a randomized controlled phase III trial which included 1,630 patients who got randomized to mFOLFOX6/CAPEOX (arm A), mFOLFOX6/CAPEOX with cetuximab (arm B) or intermittent chemotherapy (arm C). The comparison of arms A and B in patients with KRAS wild-type tumors showed an increase in ORR (57% vs. 64%, P=0.049) but no effect was observed in PFS (8.6 vs. 8.6 mo) or OS (17.9 vs. 17.0 mo) (27). The OPUS and COIN trials were pooled together in a recent ASCO presentation with a total of 423 patients with KRAS wild-type tumors and the addition of cetuximab did improve response rates (odds ratio 1.87, 95% CI, 1.07-3.28) and PFS (hazard ratio, HR 0.69, 95% CI, 0.52-0.92) but OS did not show a statistically significant improvement (HR 0.90, 95% CI, 0.73-1.11) (44). Based on these compelling results, the option for a combination of cetuximab with oxaliplatin-based chemotherapy has been removed as a recommendation from the NCCN guidelines (19) although is still recommended in parts of Europe (45).
Full Table
Panitumumab
The first trial with panitumumab explored its activity as a single agent compared to best supportive care in refractory patients. Of the 463 patients that were randomized, 57% were found to have KRAS wild-type tumors. In that population ORR was significantly improved (17% vs. 0%) along with an improvement in PFS (12.3 vs. 7.3 weeks) and OS (8.1 vs. 7.6 mo, HR 0.67, 95% CI, 0.55-0.82) compared to best supportive care (29). A randomized phase III trial (20050181) explored administering FOLFIRI with or without panitumumab as second line therapy with PFS and OS as co-primary endpoints. Of the 1,186 included patients, 597 patients had KRAS wild-type tumors. The addition of panitumumab increased ORR (35% vs. 10%), PFS (5.9 vs. 3.9 mo) and had a non-significant trend towards improved OS (14.5 vs. 12.5 mo) (30). The phase III randomized PRIME study administered FOLFOX4 as first-line therapy with or without panitumumab. Panitumumab administration significantly improved PFS (9.6 vs. 8.0 mo; P=0.02) and had a trend towards improved OS (23.9 vs. 19.7 mo, P=0.072) compared to FOLFOX4 alone with some effect on response rates although not significant (55% vs. 48%, P=0.068). A recent update to the trial is to be presented at ASCO 2013 and now shows a statistically significant improvement in OS (HR 0.78, 95% CI, 0.62-0.99) in the KRAS wild-type population who received panitumumab (46). Unlike with the 20050181 trial, the PRIME trial showed a detrimental effect when panitumumab was given to patients with KRAS mutated tumors with significantly shorter PFS (HR 1.29, P=0.02) (31). Panitumumab is licensed as first line treatment with FOLFOX outside the US only. However, both the European ESMO guidelines and NCCN guidelines do recommend panitumumab as a single agent or in combination with FOLFOX, FOLFIRI or single agent irinotecan (19,45).
Dual EGFR and VEGF monoclonal antibody inhibition
Based on strong preclinical rationale and the positive results of the BOND-2 study, a small phase II trial which randomized patients (with unknown KRAS status) to bevacizumab and cetuximab with or without irinotecan (47), two large phase III trials (48,49) explored the benefit of combining dual inhibition with either cetuximab or panitumumab with bevacizumab and standard cytotoxic chemotherapy. The phase III CAIRO-2 trial randomly assigned 755 mCRC patients previously untreated to either CAPEOX with bevacizumab or CAPEOX with bevacizumab and cetuximab. The primary endpoint for this study was PFS, and KRAS mutational status was evaluated. Cetuximab added to bevacizumab and cytotoxic chemotherapy improved response rates but had no effect on PFS or OS with increased toxicities in the KRAS wild-type population. On the other hand, addition of cetuximab had detrimental effects on the KRAS mutated population with worsening OS compared to not giving cetuximab (48). In the phase IIIB PACCE trial, the addition of panitumumab to either FOLFOX or FOLFIRI with bevacizumab was tested in 1,053 patients and led to a detriment in PFS and OS with increased toxicities in both the KRAS wild-type and KRAS mutated population (49). Cetuximab in combination with standard FOLFOX has also been explored in the adjuvant setting with results of a large phase III randomized study showing no added benefit at the expense of added toxicities (50).
EGFR inhibitors versus VEGF inhibitors: where does the data stand?
EGFR inhibitors were initially approved as single agents in chemotherapy refractory patients and it is unclear if they should be moved up to be combined with cytotoxic chemotherapy as first-line or be reserved for second or third-line therapy. Two phase II randomized clinical trials comparing the addition of panitumumab vs. bevacizumab to standard cytotoxic therapy were presented at ASCO GI in January 2013. In the PEAK study, 285 patients with KRAS wild-type mCRC were treated with modified FOLFOX6 with a PFS of 10.9 months for the group receiving panitumumab vs. 10.1 months for the group receiving bevacizumab (HR 0.87, P=0.35). Median OS had not been reached in the panitumumab group and was 25.4 months in the bevacizumab group (HR 0.72, P=0.14). Discontinuation rates were similar between the two arms (24% vs. 27%) and so were grade 3/4 adverse events (86% vs. 76%) (51). In the SPIRITT trial, 182 patients with KRAS wild-type mCRC previously treated with bevacizumab and an oxaliplatin-based regimen were randomized to FOLFIRI with panitumumab or bevacizumab as second line therapy. Median PFS [7.7 vs. 9.2 mo (HR 1.01)] and median OS [18.0 vs. 21.4 mo (HR 1.06)] were similar but response rates were higher in the panitumumab group (32% vs. 19%) (52).
CALGB 80405 is a randomized controlled trial which is comparing first-line cytotoxic chemotherapy with either cetuximab or bevacizumab (53). The results of this completed study will likely be available by the end of 2013. FIRE-3 is a randomized phase III trial comparing first-line FOLFIRI with either cetuximab or bevacizumab in mCRC and is expected to be reported at ASCO in 2013 (54). In our own institutional experience with panitumumab the total number of previous chemotherapy regimens did not significantly affect median overall survival with panitumumab suggesting that the efficacy is retained across lines of therapy, a finding consistent with other studies (55).
Liver limited disease
The role of EGFR inhibitors in liver limited disease where the goal of therapy is to convert unresectable or borderline resectable tumors to resectable disease has been explored although to a limited extent. The phase II CELIM trial investigated cetuximab in combination with either an oxaliplatin- or irinotecan-based regimen in initially unresectable patients with isolated liver metastasis (defined as ≥5 tumors, technically unresectable on the basis of inadequate functional liver remnant, infiltration of both hepatic arteries/portal vein branches or infiltration of all hepatic veins). Objective responses were seen in 68% receiving FOLFOX with cetuximab and 57% in patients receiving FOLFIRI with cetuximab. R0 resection rates were high (38% and 30% in the two groups), but as no formal comparison was performed to a group without cetuximab, the benefit of adding an EGFR inhibitor in this setting is unclear (56). Nevertheless, the elevated response rate suggests this could be a promising regimen and should be considered in patients with KRAS wild-type tumors with mCRC and liver limited disease. Similarly the CRYSTAL trial showed a modest increase in rates of surgery and R0 resection in the KRAS wild-type patients who received FOLFIRI with cetuximab versus FOLFIRI alone (surgery rate 7.9% vs. 4.6% P=0.0633; R0 resections 5.1% vs. 2.0%, P=0.0265, respectively) (25). A phase II trial reported at the annual European Society of Medical Oncology (ESMO) meeting in 2012 randomized 116 patients with KRAS wild-type tumors to mFOLFOX6 or FOLFIRI with or without cetuximab. Response rates were 66% vs. 33% in the 2 arms with improved R0 resection rates (31% vs. 9%) and a median OS of 46.6 months in the resected cetuximab arm (57).
Are all KRAS mutations equal?
Recent controversial findings suggest that not all KRAS mutations will confer resistance to EGFR inhibitor therapy. A recent retrospective study combining findings from the CRYSTAL and OPUS studies showed improved RR and PFS in patients with tumors exhibiting a codon 13 glycine to aspartate mutation (G13D) who received cetuximab compared to those who did not receive cetuximab (58). Another recent retrospective review of randomized studies with panitumumab in patients with KRAS mutated tumors did not reveal a similar benefit for adding panitumumab when looking at individual mutations in codons 12 or 13 (59). A meta-analysis looking at 7 studies with anti-EGFR agents found overall response rates to be 25.2%, 17.6% and 42.6% in codon 13 mutations vs. any other KRAS mutations vs. KRAS wild-type tumors (59). PFS was 6.4, 4.1 and 6.6 mo and OS 14.6, 11.8 and 17.3 mo for the three groups, respectively. The incidence of codon 13 mutations was 6.6% in the entire study cohort. Patients with codon 13 mutated tumors receiving EGFR inhibitor as second-line seemed to benefit more than patients receiving it in the first-line (60). It is therefore possible that tumors with G13D KRAS mutations may respond better than tumors with other KRAS mutations, although the magnitude of the benefit is small at the risk of added toxicities and cost. The NCCN guidelines do not recommend administering EGFR inhibitors to patients with codon 13D mutation based on these concerns (19). Further results from genomic analysis of the PRIME study will be presented at ASCO 2013, included analysis of KRAS exon 3, exon 4; NRAS exon 2, exon 3, exon 4; and BRAF exon 15. Findings from this study suggest that panitumumab is unlikely to benefit patients with any RAS mutations and that BRAF mutations had no predictive value (46).
Can patients who progress on one EGFR inhibitor benefit from another?
It is unclear whether panitumumab has activity in patients who have previously progressed on cetuximab (or vice versa) as two prospective studies have had discrepant results. The most important determinant for responses to subsequent panitumumab therapy from these small studies may be prior benefit from cetuximab therapy. Metges et al. reported responses in 54% of patients (N=32) on single-agent panitumumab who previously responded to cetuximab in combination with irinotecan whereas only 7.7% of patients who had no response to cetuximab with irinotecan, responded to single-agent panitumumab (61). Wadlow et al. published a phase II trial of 20 patients treated with panitumumab after progression on cetuximab, where no responses were observed, although 45% patients had stable disease with a median PFS of 1.7 months and a median OS of 5.2 months (62). Our own institutional review revealed that in patients with clinical benefit (ORR or stable disease) on cetuximab and eventual progression, 71% had subsequent clinical benefit with panitumumab therapy (55). At this time, given the limited amount of data and the lack of randomized study results, any combination of strategies for EGFR beyond progression is not recommended and this is consistent with the NCCN guidelines (19).
EGFR inhibitor toxicities—a friend or a foe?
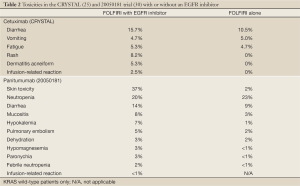
Toxicities with both EGFR inhibitors include skin rash, nail changes, fatigue, mucositis, nausea, vomiting and diarrhea as well as infusion reactions which tend to occur at a higher rate with the chimeric IgG1 monoclonal antibody cetuximab [up to 22% in areas such as North Carolina and Tennessee (63) vs. <1% with panitumumab (30)]. Toxicities seen in clinical trials when given with FOLFIRI are summarized in Table 2.
Full Table
The development of a rash is particularly common and usually occurs during the first 4 weeks of therapy. The mechanisms have been reviewed previously (64). A pooled review of toxicities in 920 patients across 10 clinical trials treated with single-agent panitumumab are presented in Table 3. Treatment-related adverse events were seen in 94% of patients with 20% experiencing a grade 3 event. Overall, 12% discontinued the drug due to toxicity. Only 4 (0.3%) patients had an infusion reaction (65).
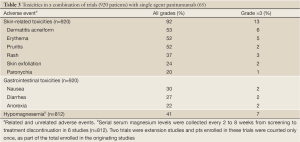
Full Table
The STEPP (Skin Toxicity Evaluation Protocol with Panitumumab) trial evaluated approaches to prevent skin toxicities in a randomized phase II trial where patients received either prophylactic or reactive skin treatment in the two arms, FOLFIRI with panitumumab (6 mg/kg every 2 weeks) vs. irinotecan with panitumumab (9 mg/kg every 3 weeks). The prophylactic skin treatment consisted of using a skin moisturizer, sunscreen, 1% hydrocortisone cream and doxycycline twice daily. The grade ≥2 skin toxicities were reduced by more than 50% in the prophylactic group compared to the reactive treatment group (29% vs. 62%) (64).
Electrolyte disturbances, especially low magnesium and/or low calcium, believed to be due to EGFR blockade in the kidney, can occur and can be seen for up to 8 weeks after discontinuing treatment. Monitoring is therefore required and recommended for up to 8 weeks after therapy and repletion of electrolytes may be needed (17,21).
The first trials conducted with EGFR inhibitor therapy did recognize that skin rash seem to be associated with improved efficacy. The development and grade of rash had been associated with an improved OS in both cetuximab and panitumumab studies. For example, Peeters et al. found patients with grade 2-4 skin toxicities to have a significantly longer OS (7.9 vs. 5.6 mo; hazard ratio 0.60, P=0.0033) compared to patients with grade 1 skin toxicities. In this study 91% of patients had grade 1 or higher skin toxicity with 69% having grade 2-4 (66).
The EVEREST phase I/II study randomized irinotecan-refractory patients who had not developed a rash > grade 1 after 21 days of standard-dose cetuximab (400 mg/m2 initial dose, then 250 mg/m2 per week) plus irinotecan, to dose escalations versus continuing the same dose. Of 157 patients, 89 patients were randomized after 21 days. The dose escalation was consistent with higher drug pharmacokinetics [Cmax and area under curve (AUC)] and was associated with an increase in skin reactions ≥ grade 2. In the KRAS-wild-type population, response rates were 43% in the dose escalation vs. 30% in the same dose population (compared to 42% in the patients who had a rash with the initial dosing) but PFS and OS were not markedly different. Grade 2/3 skin reactions, diarrhea, hypomagnesemia and dry skin were more frequent in the dose escalation group but infusion reactions were not increased (67).
Cetuximab is associated with infusion reactions, particularly in North Carolina and Tennessee where grade 3-4 hypersensitivity reactions were reported in up to 22%, all of them occurring during the first infusion (63). This is thought to be linked to IgE specific for galactose-alpha-1.3-galactose in these individuals and may be caused by a crossreaction with a specific antigen, possibly related to animals or plants, found in those regions (68). Other areas have found a lower incidence with grade 3 or 4 infusion reactions being reported in 2.3% of patients in the CRYSTAL trial (25). Panitumumab, being a fully humanized monoclonal antibody, causes infusion reactions in <1% (30).
Mechanisms of resistance
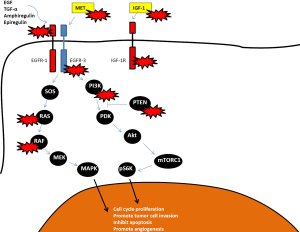
Mutations in the KRAS gene cause resistance to EGFR inhibition, as the MAPK pathway remains constitutively active even in the presence of an EGFR inhibitor. It is not clear why only 40-60% (10-20% response rate, 30-40% stable disease) of patients with KRAS wild-type tumors benefit from EGFR inhibition. Furthermore, even in the presence of a response, progression eventually occurs. Several mechanisms of resistance have been proposed (see Figure 2).
Several investigators have looked at predictive factors for EGFR inhibitor responses. PIK3CA mutations and PTEN loss occur in ~15% and 20% of mCRC tumors and result in constitutive activation of the PIK3/Akt/mTor pathway which is an important anti-apoptotic and pro-survival tumor cell pathway (69). Activators of the PI3K/Akt/mTor pathway include IGF-R1, IGF-2, Her-2, Her-3 and Her-4 receptors as well as the MAP kinase pathway with crosstalk between the two pathways. PIK3CA mutations and/or PTEN loss have been shown to predict response in some (70-73) but not all studies (74,75). These studies are all limited by small numbers and often a lack of validation for correlative testing. The largest study to date with 1,022 tumor samples showed only PIK3CA mutations in exon 20 (constituting the kinase domain) to be predictive of response but not mutations in exon 9 (the helical domain). About 20% of PI3KCA mutations were located in exon 20 while 68.5% were located in exon 9. The investigators did not look at PTEN expression (76). These results suggest that alterations in the PIK3/Akt/mTor pathway may be responsible for some of the patients who do not respond to EGFR inhibitors initially.
A few groups have specifically looked at mechanisms of resistance in patients who have progressed on an EGFR inhibitor. Montagut et al. found two out of 10 patients who had progressed on cetuximab to have a mutation in the EGFR ectodomain (S492R) which prohibits binding of cetuximab but not panitumumab (77). Misale et al. performed KRAS gene deep sequencing on tumors from patients who had progressed on an EGFR inhibitor and found secondary KRAS mutations in 6 out of 10 cases suggesting that this could either be acquired mutations on therapy or the selection of pre-existent KRAS mutant clones (78).
IGF-1R is upregulated in 50-90% of mCRC and has been associated with poor prognosis. Cells with an altered IGF-1R pathway seem to escape EGFR inhibitor mediated cell death by activation of the PI3K pathway by heterodimerization of IGF-1R with EGFR. Overexpression of IGF-1 has been associated with resistance to cetuximab in KRAS wild-type tumors (79). HER3 is overexpressed in 30-80% of metastatic CRC and has been associated with EGFR inhibitor resistance (80). Its effects are mediated through the PI3K/Akt pathway. MET overexpression is found in most mCRC, both in KRAS wild-type and KRAS mutant tumors and interacts with the EGFR pathway to promote growth of CRC cells (81). Preclinical evidence suggests that coupling of MET with HER3 may lead to sustained activation of PI3K/Akt pathway in lung cancer cell lines, thereby bypassing the inhibited EGFR (82).
Furthermore, it is possible that resistance to EGFR inhibitors could result from a selection of clones already resistant to the drugs. It is therefore clear that several different mechanisms may signal resistance through the PI3K/Akt pathway and extend survival of the cancer cell. This is currently an active area of ongoing research.
Summary
EGFR inhibitors are an important addition to the growing armamentarium in metastatic colorectal cancer. In an era of emphasis on refining therapy, the presence of KRAS mutation will predict for resistance and limit exposure to patients who are more likely to benefit. Although this is a great step forward, more work needs to be done to select better patients who will definitely benefit from this expensive and potentially toxic therapy. The presence of BRAF mutations does not seem to fulfill this predictive value. Studies to elucidate further the role of positive predictive markers are ongoing. Additionally, we need to deepen our understanding of the mechanisms that drive resistance to EGFR inhibitors to further refine selection and improve outcome. Agents that are thought to reverse resistance to EGFR inhibitors such as those targeting PI3K, c-MET, Her-3 or IGF-1R are currently under study.
EGFR inhibitors have exhibited single agent activity, and seem to synergize very well with standard chemotherapy except for cetuximab and FOLFOX. Preliminary data suggests that EGFR inhibitors have similar effectiveness to VEGF inhibitors when combined with standard chemotherapy, with the definitive results from large randomized studies (FIRE-3, CALGB 80405) eagerly anticipated. Evidence suggests that strategies to combine EGFR and VEGF inhibitors in the first line setting can be detrimental to outcome. Skin toxicity remains the main limiting factor for the utilization of EGFR inhibitors, but strategies including the use of agents such as minocycline or doxycycline added to topical care seem to limit the severity of the rash.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [PubMed]
- Gray BN. Colorectal cancer: the natural history of disseminated disease-a review. Aust N Z J Surg 1980;50:643-6. [PubMed]
- Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008;26:5721-7. [PubMed]
- Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995;19:183-232. [PubMed]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74. [PubMed]
- Messa C, Russo F, Caruso MG, et al. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol 1998;37:285-9. [PubMed]
- Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol 2000;21:105-15. [PubMed]
- Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer 2001;92:1331-46. [PubMed]
- Mayer A, Takimoto M, Fritz E, et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer 1993;71:2454-60. [PubMed]
- Spano JP, Lagorce C, Atlan D, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 2005;16:102-8. [PubMed]
- Resnick MB, Routhier J, Konkin T, et al. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin Cancer Res 2004;10:3069-75. [PubMed]
- Baselga J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur J Cancer 2001;37:S16-22. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005;7:301-11. [PubMed]
- Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007;25:3712-8. [PubMed]
- Sunada H, Magun BE, Mendelsohn J, et al. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A 1986;83:3825-9. [PubMed]
- Squibb B-M. Erbitux Package Insert. Available online: http://packageinserts.bms.com/pi/pi_erbitux.pdf, Rev March 2013.
- Pfeiffer P, Nielsen D, Bjerregaard J, et al. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol 2008;19:1141-5. [PubMed]
- Network NCC. NCCN clinical practice guidelines in oncology (NCCN Guidelines): colon cancer. Version 3. 2013. Accessed 04/25/13.
- Yang XD, Jia XC, Corvalan JR, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999;59:1236-43. [PubMed]
- Inc. A. Vectibix Package Insert. Available online: http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf, Rev March 2013.
- Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5. [PubMed]
- Brink M, de Goeij AF, Weijenberg MP, et al. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis 2003;24:703-10. [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [PubMed]
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. [PubMed]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Barber TD, Vogelstein B, Kinzler KW, et al. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med 2004;351:2883. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 2005;23:1803-10. [PubMed]
- Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res 2010;16:2205-13. [PubMed]
- Jiang Z, Li C, Li F, et al. EGFR gene copy number as a prognostic marker in colorectal cancer patients treated with cetuximab or panitumumab: a systematic review and meta analysis. PLoS One 2013;8:e56205. [PubMed]
- Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9:962-72. [PubMed]
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy? Genet Med 2013;15:517-27. [PubMed]
- Han CB, Li F, Ma JT, et al. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: a meta-analysis and systematic review. Cancer Invest 2012;30:741-7. [PubMed]
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Langer C, Kopit J, Awad M, et al. Analysis of K-RAS mutations in patients with metastatic colorectal cancer receiving cetuximab in combination with irinotecan: results from the EPIC trial. Ann Oncol 2008;19:133.
- Taieb J, Maughan TS, Bokemeyer C, et al. Cetuximab combined with infusional 5-fluorouracil/folinic acid (5-FU/FA) and oxaliplatin in metastatic colorectal cancer (mCRC): a pooled analysis of COIN and OPUS study data. J Clin Oncol 2012;30:abstr 3574.
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. [PubMed]
- Oliner K, Douillard J, Siena S, et al. Analysis of KRAS/NRAS and BRAF mutations in the phase III PRIME study of panitumumab (pmab) plus FOLFOX versus FOLFOX as first-line treatment (tx) for metastatic colorectal cancer (mCRC). Available online: http://www.asco.org/sites/www.asco.org/files/abstract_115136.pdf, 2013.
- Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 2007;25:4557-61. [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [PubMed]
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672-80. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Schwartzberg L, Rivera F, Karthaus M, et al. PEAK (study 20070509): A randomized phase II study of mFOLFOX6 with either panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) in patients (pts) with unresectable wild type (WT) KRAS metastatic colorectal cancer (mCRC). J Clin Oncol 2012;30:abstr 446.
- Hecht J, Cohn A, Dakhil S, et al. SPIRITT (study 20060141): A randomized phase II study of FOLFIRI with either panitumumab (pmab) or bevacizumab (bev) as second-line treatment (tx) in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC). J Clin Oncol 2012;30:abstr 454.
- Venook AP, Blanke CD, Niedzwiecki D, et al. Revisiting the Cancer and Leukemia Group B/Southwest Oncology Group 80405 Trial: a phase III trial of chemotherapy and biologic agents for patients with untreated advanced colorectal adenocarcinoma. Clin Colorectal Cancer 2007;6:536-8. [PubMed]
- ClinicalTrials.gov. 5-FU, folinic acid and irinotecan (FOLFIRI) plus cetuximab versus FOLFIRI plus bevacizumab in first line treatment colorectal cancer (CRC). Available online: http://clinicaltrials.gov/ct2/show/NCT00433927, Accessed 05/08/2013.
- Haraldsdottir S, Rose JS, Wu C, et al. A single institutional experience with panitumumab in metastatic colorectal cancer. Journal of Cancer Therapy 2012;3:948-55.
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet oncol 2010;11:38-47. [PubMed]
- Xu J, Ye L, Ren L, et al. A randomized, controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastasis. ESMO (ed).2012:abstract 1806.
- Tejpar S, Celik I, Schlichting M, et al. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol 2012;30:3570-7. [PubMed]
- Peeters M, Douillard JY, Van Cutsem E, et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol 2013;31:759-65. [PubMed]
- Chen J, Ye Y, Sun H, et al. Association between KRAS codon 13 mutations and clinical response to anti-EGFR treatment in patients with metastatic colorectal cancer: results from a meta-analysis. Cancer Chemother Pharmacol 2013;71:265-72. [PubMed]
- Metges J, Raoul J, Achour N, et al. PANERB study: panitumumab after cetuximab-based regimen failure. J Clin Oncol 2010;28:e14000.
- Wadlow RC, Hezel AF, Abrams TA, et al. Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab. Oncologist 2012;17:14. [PubMed]
- O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 2007;25:3644-8. [PubMed]
- Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351-7. [PubMed]
- Peeters M, Van Cutsem E, Berlin J, et al. Safety of panitumumab, a fully human monoclonal antibody against the epidermal growth factor receptor (EGFr), in patients (pts) with metastatic colorectal cancer (mCRC) across clinical trials. J Clin Oncol 2007;25:4138.
- Peeters M, Siena S, Van Cutsem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 2009;115:1544-54. [PubMed]
- Van Cutsem E, Tejpar S, Vanbeckevoort D, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol 2012;30:2861-8. [PubMed]
- Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008;358:1109-17. [PubMed]
- Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004;304:554. [PubMed]
- Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009;69:1851-7. [PubMed]
- Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 2007;97:1139-45. [PubMed]
- Loupakis F, Pollina L, Stasi I, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol 2009;27:2622-9. [PubMed]
- Perrone F, Lampis A, Orsenigo M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol 2009;20:84-90. [PubMed]
- Prenen H, De Schutter J, Jacobs B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res 2009;15:3184-8. [PubMed]
- Souglakos J, Philips J, Wang R, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer 2009;101:465-72. [PubMed]
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010;11:753-62. [PubMed]
- Montagut C, Dalmases A, Bellosillo B, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012;18:221-3. [PubMed]
- Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012;486:532-6. [PubMed]
- Scartozzi M, Mandolesi A, Giampieri R, et al. Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. Int J Cancer 2010;127:1941-7. [PubMed]
- Scartozzi M, Mandolesi A, Giampieri R, et al. The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab. Oncologist 2011;16:53-60. [PubMed]
- Seiden-Long IM, Brown KR, Shih W, et al. Transcriptional targets of hepatocyte growth factor signaling and Ki-ras oncogene activation in colorectal cancer. Oncogene 2006;25:91-102. [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [PubMed]