The rise of the FGFR inhibitor in advanced biliary cancer: the next cover of time magazine?
Introduction
Cholangiocarcinomas (CCA) are heterogeneous epithelial tumors arising from the biliary tree with features of cholangiocyte differentiation (1). CCA is the most common primary biliary tract malignancy and the second most common primary hepatic malignancy (1,2). Overall, CCAs account for 3% of all gastrointestinal malignancies (2). The incidence of CCA has increased over the past three decades; however, the 5-year survival remains dismal at approximately 10% (3).
Based on their anatomic location within the biliary tree, CCAs are classified into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) subtypes (4). pCCA arises from the large bile ducts in the hepatic hilum. Proximally, pCCA is separated anatomically from the iCCA by the second-order bile ducts, and distally the cystic duct insertion serves as the point of distinction between pCCA and dCCA (5). In addition to having different anatomic origins, these subtypes also have distinct epidemiology, risk factors, pathogenesis, and treatment (1). pCCA is the most common subtype; in a large series of 564 patients with biliary tract cancer, 50% had pCCA, 42% had dCCA, and 8% had iCCA (6).
The prognosis of CCA is dismal with medial survival of less than 2 years in patients with disease not amenable to surgical resection (7). Potentially curative surgery/transplantation is an option only in early-stage disease. Patients with iCCA have the best outcome following surgical resection; the 5-year survival after R0 resection was 63% for iCCA, 30% for dCCA, and only 27% for pCCA (6). Neoadjuvant chemoradiation followed by liver transplantation is a consideration for a subset of pCCA patients who meet stringent criteria (1). In carefully selected patients, this approach has resulted in 2- and 5-year recurrence free survival rates of 78% and 65%, respectively (8).
For patients with advanced disease who are not candidates for either surgical resection or liver transplantation, systemic chemotherapy with gemcitabine and cisplatin is the practice standard (9). The combination of gemcitabine-cisplatin confers a modest survival advantage with an overall median survival of 11.7 months compared to 8.1 months with gemcitabine alone (9).
CCAs are heterogeneous tumors and medical therapy tailored to the features of each individual tumor is a promising approach. However, no targeted molecular therapies have been approved for use in biliary tract cancer. Genetic factors in CCA include chromosomal aberrations, genetic and epigenetic alterations in tumor suppressor genes and oncogenes. A recent molecular characterization of 260 biliary tract cancers identified 32 significantly altered genes, including potentially targetable genetic alterations in 40% of cases (10). Mutations in ARID1B, ELF3, PRKACA and PRKACAB occurred preferentially in pCCA and dCCA, whereas, mutations in isocitrate dehydrogenase 1 and 2 (IDH 1/2), EPHA2 and BAP1 were more frequently noted in iCCAs (10). Fibroblast growth factor receptor (FGFR) gene fusions were noted exclusively in iCCAs. KRAS, SMAD4, ARID1A, and GNAS mutations were noted in all three CCA subtypes (10). KRAS and SMAD4 mutations were noted in a whole-exome sequencing analysis of eight liver-fluke related CCAs (11). This study identified 206 mutations in 187 genes including TP53 mutations in 44.4% of cases, KRAS mutations in 16.7% of cases, SMAD4 in 16.7% of cases. In addition, somatic mutations in ten newly implicated genes including MLL3, ROBO2, GNAS, and RNF43 were identified (11). In another recent whole-exome sequencing analysis, TP53 mutations were noted to occur more frequently in liver fluke related CCA, while somatic mutations in BAP1 and ARID1A, IDH1, and IDH2 occurred with increased frequency in non-liver fluke related CCA (12). BAP1 and ARID1A are chromatin-remodeling genes and inactivating mutations of these genes were also identified in exome sequencing of 32 iCCAs (13).
Deregulation of growth factor tyrosine kinases, noted in various malignancies including CCA, plays a critical role in tumor initiation and progression. These include the FGFR pathway, ERBB family of receptor tyrosine kinases including epidermal growth factor receptor (EGFR), and hepatocyte growth factor (HGF) receptor. EGFR activation leads to activation of p44/42 mitogen-activated protein kinases (MAPKs), which has a well-established oncogenic role (14). ERBB2 overexpression, another ERBB family member, has been associated with development of biliary tract cancer in preclinical studies (15). HGF, a stroma-derived paracrine mediator and ligand for the MET receptor, promotes tumor invasiveness and metastasis (16,17). Aberrant overexpression of HGF and MET occurs in CCA and is associated with a poor prognosis (18,19).
Fibroblast growth factor (FGF) pathway
The FGF pathway consists of 22 human FGFs and four transmembrane receptor tyrosine kinases, FGFR 1–4 (20-22). FGF signaling is involved in a myriad of biological processes including regulation of developmental pathways and mesodermal patterning of the embryo, physiological functions such as regulation of angiogenesis and wound repair, and regulation of essential cell behaviors including proliferation, differentiation, survival, migration, and angiogenesis (23,24). The FGF-FGFR axis is activated with binding of FGF to FGFR and heparin sulphate proteoglycan in a specific complex on the surface of the cell (25). In this complex, a central heparin molecule links two FGFs into a dimer that bridges two FGFR chains (25). FGFR dimerization is homo-dimer driven. Once formed, this complex activates the FGFR tyrosine kinase with resultant autophosphorylation of tyrosines in the C-terminus, kinase insert, and juxtamembrane region. Phospho-FGFR then phosphorylates adapter proteins including FGFR substrate 2 and 3. This leads to activation of various intracellular signaling cascades involved in promotion of cell survival and proliferation including Ras-MAPK, phosphatidylinositol 3-kinase (PI3K)-protein kinase Akt/protein kinase B pathways, p90 ribosomal protein S6 kinase 2, signal transducers and activators of transcription, and Src (24,26).
The ubiquitous role of FGF signaling in various biological processes integral to cell survival increases susceptibility to oncogenic transformation with aberrant FGF signaling (24). Deregulated FGF signaling mediates carcinogenesis by enhancing cellular proliferation, migration, survival, and invasion and promoting tumor angiogenesis (24). Several different mechanisms underlie the oncogenic potential of FGF signaling. Genomic alteration of FGFR can result from activating mutations, receptor gene amplifications, and chromosomal translocations (24). Intragenic translocations can lead to formation of a fusion protein consisting of a transcription factor fused to an FGFR kinase domain with consequent FGFR dimerization and activation (24,27,28). Genomic aberrations lead to ligand-independent FGF signaling. Ligand-dependent signaling can also promote tumor formation and progression through autocrine or paracrine production of ligand from cancer or stromal cells respectively (24). Lastly, tumor angiogenesis is augmented by cross-talk between FGF ligands, vascular endothelial growth factors, and inflammatory mediators in the tumor stroma. Preclinical studies have demonstrated that FGFs and VEGFs can act in a synergistic manner to enhance tumor angiogenesis (29).
FGFR signaling and gene fusions in CCA
Aberrant FGF signaling has also been implicated in CCA carcinogenesis. FGFR 1, 2, and 4 are upregulated in CCA cell lines (30). An autocrine, feed-forward pathway between the oncogenic hippo signaling pathway and FGFR signaling has recently been reported. In this pathway, yes-associated protein (YAP), a transcriptional co-activator in oncogenic hippo signaling, upregulates FGFR 1, 2 and 4 (30). In turn, FGFR2 stimulation by FGF5 upregulates YAP (30). Pan-FGFR inhibition with BGJ398 promoted cell death in CCA cells and significantly reduced tumor burden in a YAP-driven genetic murine model of CCA as well as a patient-derived xenograft model with enhanced YAP expression (30). These observations indicate that FGFR inhibition has strong therapeutic potential in a subset of CCAs with activation of oncogenic hippo signaling. FGF has also been shown to promote migration of CCA cells via phosphorylation of MEK 1/2 (31). An angiogenic role of FGF signaling has also been described in CCA cells (32). Abundant FGFR4 expression occurs in all three subtypes and is associated with a poor prognosis of pCCA and iCCA (33). Specifically, FGFR4 has been reported to induce proliferation, invasion and epithelial-mesenchymal transition of CCA cells (33). Accordingly, FGFR inhibition with AP24354 promoted apoptosis while inhibiting CCA cell proliferation and invasion (33).
Gene sequencing enables discovery of “actionable” therapeutic target genes in patients with malignancy (34). Identification of “driver” target mutations may have a higher therapeutic impact. Gene fusions are an important class of driver mutations that play a vital role in certain cancer (34). For instance, the BCR-ABL fusion gene mutation is critical in chronic myeloid leukemia, and these patients tend to respond very well to imatinib, a small-molecule kinase inhibitor. The significant role of gene fusions/translocations in epithelial cancers has become increasingly evident. Several recent studies have identified FGFR2 gene fusions in CCA (34-38). Wu et al. reported the FGFR-BICCI gene fusion in two cases of CCA (34). Subsequently, in a cohort of 66 iCCA cases, the FGFR2 gene fusion was detected in 13.6% of cases (38). In addition to reporting several FGFR-2BICC1 fusions, a novel gene fusion, FGFR2-AHCYL1, was also identified in this series (38). KRAS and BRAF mutations were not present in CCA patients with FGFR2 gene translocations, signifying the potential of these gene fusions as driver mutations. A similar prevalence of FGFR2 gene fusions in iCCA was reported in a North American cohort; utilizing fluorescence in situ hybridization, Graham et al. identified FGFR2 gene fusions in 12/96 (13%) iCCA cases (36). Integrated genome-wide and whole transcriptome sequence analyses in six patients with advanced CCA identified recurrent translocations events involving the FGFR2 locus in three of six patients (37). Genomic profiling in another cohort of iCCAs identified FGFR2 gene fusions in 3 of 28 cases, including one case each of FGFR-BICC1 fusion and FGFR2-TACC3 fusion, and a novel fusion, FGFR2-KIAA 1598 (35).
Wu et al. proposed that oligomerization may serve as the common mechanism of activation of FGFR fusion proteins as a majority of the FGFR fusion partners had domains with dimerization motifs (34). Indeed, mechanistic studies demonstrated that the fusion domains provided by the FGFR binding partners interacted in vitro as oligomerization domains, supporting the notion that these partners mediate oligomerization which initiate activation of the respective FGFR kinase in tumors with the FGFR translocations (34).
Characteristics of CCA patients with FGFR2 gene fusions
From a histological standpoint, prominent intraductal cancer growth and anastomosing tubular glands with desmoplasia are present in iCCAs harboring FGFR2 translocations (36). The majority of the tumors with FGFR2 gene fusions had significantly diminished expression of cytokeratin 19, a typical marker of pancreaticobiliary tumors (36). Interestingly, the presence of FGFR2 gene translocations was associated with enhanced survival (123 vs. 37 months) in a North American study, suggesting that the presence of FGFR2 gene fusions may have prognostic utility (36). Moreover, CCA patients with FGFR2 gene fusions were younger (median age of 52 vs. 65 years) with a female preponderance (13% vs. 4%). However, in an Asian cohort of CCA patients with FGFR2 gene fusions, no survival or gender differences were noted (38). An association between hepatitis B and C virus infection and cases with FGFR2 translocations was noted in this latter cohort, whereas such an association was absent in the North American cohort. The geographic differences with distinct risk factors and etiologies likely account for the discrepancies in patient characteristics and outcomes between these two studies. FGFR2 translocations were noted in patients with de novo CCA rather than those with preexisting primary sclerosing cholangitis (PSC) (36). The only CCA subtype in which FGFR2 gene fusions have been identified is the iCCA subtype. Thus, these etiological associations are not surprising as risk factors such as hepatitis B and C are typically associated with iCCA, whereas preexisting PSC is noted mainly in pCCA patients.
In several of the cohorts, FGFR2 gene fusions were detected in resected, surgical specimens, indicating an earlier clinical stage (39). This suggests that FGFR2 translocations are an early event in carcinogenesis, and hence may serve as driver events in CCA (39).
FGFR as a therapeutic target in CCA
FGFR-selective small molecule kinase inhibitors (SMKIs)
Preclinical studies have demonstrated that only cells harboring the FGFR gene fusions were sensitive to FGFR inhibitors (34,38), indicating a role for targeted FGFR kinase inhibition in tumors harboring these fusions. Indeed, FGFR genetic aberrations appear to be the most important predictor for sensitivity to NVP-BGJ398, a pan-FGFR inhibitor (40). In vitro expression of the FGFR2 fusion kinases activates the MAPK pathway and promotes anchorage-independent growth (38). Moreover, subcutaneous transplantation of fusion kinase expressing cells resulted in tumorigenesis in vivo. These effects have been attributed to the kinase activity of the fusion kinases (38). Hence, therapeutic targeting of FGFR with SMKIs has emerged as a promising individualized approach in patients harboring FGFR2 fusions (Table 1). NVP-BGJ398 and ARQ 087 are SMKIs with specificity against FGFRs. Preclinical studies have demonstrated suppression of downstream MAPK signaling and oncogenic activity of FGFR fusion kinases with these FGFR-selective SMKIs (38). Clinical efficacy of BGJ398 is currently being investigated in a phase II study of adult patients with advanced or metastatic CCA with FGFR gene fusions or another FGFR genetic aberration (Table 1). ARQ 087 is an FGFR inhibitor with potent activity against FGFR1, 2, and 3. It is currently under clinical investigation in a phase I trial of patients with advanced solid tumors with FGFR genetic alterations including patients with iCCA harboring FGFR2 gene fusions (Table 1). JNJ-42756493 is an oral pan-FGFR selective SMKI (41). In a phase I trial of solid tumors with genetic FGFR aberrations including amplifications, mutations, and translocations, JNJ-42756493 therapy resulted in four confirmed responses and one unconfirmed partial response in patients with glioblastoma and urothelial and endometrial cancer, while 16 patients had stable disease (41). In this early phase study, the 36 patients without known FGFR alterations did not have any significant response (41). TAS-120 is a small molecule selective irreversible FGFR inhibitor being investigated in a phase I trial of patients with advanced solid tumors or multiple myeloma with or without FGF/FGFR-related abnormalities (Table 2). AZD4547 is a pan-FGFR inhibitor with demonstrated efficacy against several FGRF-driven cancers in preclinical studies (42). AZD4547 is currently being investigated in several clinical trials.

Full table
Full table
CH5183284/Debio 1347 is an orally available inhibitor with selectivity against FGFR1, FGFR2, and FGFR3. CH5183284/Debio 1347 demonstrated antitumor activity against cancer cell with various FGFR genetic alterations in preclinical models including cell lines and xenograft models (43). CH5183284/Debio 1347 is currently under phase I clinical investigation in solid organ malignancy patients whose tumors have a genetic aberration of FGFR1, 2, or 3 (Table 2).
Non-selective SMKIs
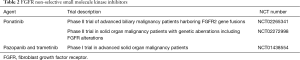
Directed therapy with ponatinib, a non-selective pan-FGFR inhibitor, decreased serum levels of carbohydrate antigen 19-9 (CA 19-9), a CCA tumor marker, and induced tumor necrosis in an advanced iCCA patient with the FGFR-MGEA5 fusion (37). Further evidence of antitumor activity of FGFR inhibition was noted in an FGFR-TACC3 fusion-positive patient initially treated with pazopanib, another non-selective SMKI, with some tumor regression. After progression on pazopanib, ponatinib therapy resulted in stabilization of disease in this patient with advanced CCA (37). This promising preliminary evidence has served as the premise for several clinical studies prospectively investigating SMKIs of FGFR. Ponatinib is being assessed in a phase II study of advanced biliary cancer harboring FGFR2 gene fusions detected by either next-generation sequencing or break-apart FISH (Table 2). Another ongoing phase II trial is assessing the efficacy of ponatinib in advanced malignancies including CCA with genetic aberrations of FGFR including mutations, fusions, and/or amplifications (Table 2). Taking into account the heterogeneous nature of CCA, one can envision the advantage of combinatorial therapeutics. KRAS mutations are a frequent occurrence in CCA and one of the downstream effector pathways of KRAS is the Raf/MEK/ERK pathway (2). A phase II study of the MEK inhibitor, trametinib, in advanced biliary tract cancer revealed some benefit although most patients had progressive disease (ClinicalTrials.gov identifier: NCT01943864). The combination of pazopanib and trametinib is currently being assessed in a phase I trial of patients with advanced solid tumors including CCA (Table 2).
Antibody therapy
Monoclonal antibodies directed against FGFR2 have exhibited tumor suppressive potential in preclinical CCA models (44). The FGFR genes encode multiple structural variants through tissue-specific splicing. The FGFR2-IIIb isoform is found selectively in epithelial cells, whereas the FGFR2-IIIc isoform has selectivity for mesenchymal cells (45). RNASeq data from one study detected the FGFR2-IIIb isoform in all identified fusions (37). The FGFR2-IIIB isoform has been shown to have binding specificity for the FGF7 and FGF10 ligands. This selectivity has clinical significance as FGFR2-IIIb specific antibodies would avoid the off-target effects of SMKIs and hence would represent an attractive chemotherapeutic option in fusion-positive cases of advanced CCA. The combination of a FGFR2-IIIb specific monoclonal antibody and an FGFR SMKI would be another attractive option, one with the potential of attaining more complete FGFR signaling inhibition (39). FPA144 is a humanized monoclonal antibody directed against the FGFR2b isoform. FPA144 has demonstrated robust efficacy in preclinical tumor models of gastric cancer and is currently being assessed in a phase I clinical trial in patients with advanced solid tumors with FGFR2b overexpression or FGFR2 amplification (ClinicalTrials.gov identifier: NCT02318329).
Heat shock protein inhibitors
Heat shock protein 90 (HSP90) is a molecular chaperone which regulates the maturation and functional stability of a myriad of cellular proteins, including key regulators of cell proliferation, differentiation, and survival (46). Cancer cells can subvert this essential regulatory function to facilitate malignant transformation (46). Selective targeting of HSP90 using small molecule inhibitors has been shown to be a valid chemotherapeutic approach in fusion-driven lung cancer and represents an alternative to direct kinase inhibition (47). HSP90 and its co-chaperone CDC37 are essential in the stability of many oncogenic proteins. FGFR1OP2 gene encodes a protein of unknown function known as FGFR1 oncogene partner 2 (FOP2). The fusion protein FOP2-FGFR1 has constitutive tyrosine kinase activity. HSP90-CDC37 forms a permanent complex with FOP2-FGFR1 which protects the fusion protein from degradation and holds it in a permanently active conformation in a leukemic cell line (48). Inhibition of HSP90 function also reduces the signaling capacity of FGFR3 and induces its degradation (49). In bladder cancer harboring the FGFR3-TACC3 fusion, ganetespib, a selective HSP90 inhibitor, induced loss of expression of the fusion protein and inhibited several oncogenic signaling proteins, and induction of apoptosis (50). The combination of ganetespib and BGJ398 had enhanced efficacy compared to either agent alone. These data suggest that HSP90 inhibition may not only be an alternative but a potentially complementary approach to kinase inhibition in fusion-drive malignancies (50).
Future directions
FGFR inhibition has emerged as a viable therapeutic option in advanced biliary tract cancer particularly in patients with FGFR2 gene fusions. However, despite the purported FGFR selectivity of several newly developed inhibitors, multi-kinase activity of these inhibitors has been observed (51). The resultant off-target toxicities are a significant limitation. Future efforts should be directed at development of FGFR specific kinase inhibitors (and even FGFR2 selective SMKIs for patients with FGFR2 fusions) with minimal activity against other kinases such as vascular endothelial growth factor receptors and platelet derived growth factor receptors. Accordingly, further studies are needed to assess the efficacy of combinatorial approaches which would accomplish more complete blockade of the FGFR signaling axis. For instance, the combination of a FGFR specific monoclonal antibody and FGFR SMKI has the potential to attain comprehensive blockade of FGFR signaling. Approaches which would target FGFR signaling as well as pathways downstream of FGFR such as the PI3K-Akt-mTOR pathway also hold significant promise. Further work is also needed to assess the antitumor potential of HSP90-CDC37 inhibition in biliary tract cancer. Currently, the focus of novel clinical investigation in CCA is directed primarily at molecular aberrations present in subsets of CCA patients. However, dual-target inhibition appears to be a more viable approach than targeting individual molecular aberrations given the heterogeneous nature of CCA.
Acknowledgements
We thank Ms. Courtney Hoover for excellent secretarial support.
Funding: This work was supported by National Institutes of Health grants T32DK007198 (SI Ilyas), K12 CA90628 (MJ Borad), NIH/NCI 1DP2CA195764 (MJ Borad) and the Mayo Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29. [Crossref] [PubMed]
- Rizvi S, Borad MJ, Patel T, et al. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin Liver Dis 2014;34:456-64. [Crossref] [PubMed]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134-44. [Crossref] [PubMed]
- Rizvi S, Gores GJ. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion 2014;89:216-24. [Crossref] [PubMed]
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22. [Crossref] [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [Crossref] [PubMed]
- Farley DR, Weaver AL, Nagorney DM. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc 1995;70:425-9. [Crossref] [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 2012;44:690-3. [Crossref] [PubMed]
- Chan-On W, Nairismägi ML, Ong CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 2013;45:1474-8. [Crossref] [PubMed]
- Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470-3. [Crossref] [PubMed]
- Yoon JH, Gwak GY, Lee HS, et al. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol 2004;41:808-14. [Crossref] [PubMed]
- Kiguchi K, Carbajal S, Chan K, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res 2001;61:6971-6. [PubMed]
- Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer 2006;119:477-83. [Crossref] [PubMed]
- Nakamura T, Matsumoto K, Kiritoshi A, et al. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res 1997;57:3305-13. [PubMed]
- Lai GH, Radaeva S, Nakamura T, et al. Unique epithelial cell production of hepatocyte growth factor/scatter factor by putative precancerous intestinal metaplasias and associated "intestinal-type" biliary cancer chemically induced in rat liver. Hepatology 2000;31:1257-65. [Crossref] [PubMed]
- Miyamoto M, Ojima H, Iwasaki M, et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br J Cancer 2011;105:131-8. [Crossref] [PubMed]
- Sandhu DS, Baichoo E, Roberts LR. Fibroblast growth factor signaling in liver carcinogenesis. Hepatology 2014;59:1166-73. [Crossref] [PubMed]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol 2001;2:REVIEWS3005.
- Johnson DE, Lu J, Chen H, et al. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol 1991;11:4627-34. [Crossref] [PubMed]
- Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets 2009;9:639-51. [Crossref] [PubMed]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010;10:116-29. [Crossref] [PubMed]
- Pellegrini L, Burke DF, von Delft F, et al. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 2000;407:1029-34. [Crossref] [PubMed]
- Gotoh N, Laks S, Nakashima M, et al. FRS2 family docking proteins with overlapping roles in activation of MAP kinase have distinct spatial-temporal patterns of expression of their transcripts. FEBS Lett 2004;564:14-8. [Crossref] [PubMed]
- Xiao S, Nalabolu SR, Aster JC, et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t(8;13) leukaemia/lymphoma syndrome. Nat Genet 1998;18:84-7. [Crossref] [PubMed]
- Roumiantsev S, Krause DS, Neumann CA, et al. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell 2004;5:287-98. [Crossref] [PubMed]
- Presta M, Dell'Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev 2005;16:159-78. [Crossref] [PubMed]
- Rizvi S, Yamada D, Hirsova P, et al. A Hippo and Fibroblast Growth Factor Receptor Autocrine Pathway in Cholangiocarcinoma. J Biol Chem 2016;291:8031-47. [Crossref] [PubMed]
- Narong S, Leelawat K. Basic fibroblast growth factor induces cholangiocarcinoma cell migration via activation of the MEK1/2 pathway. Oncol Lett 2011;2:821-5. [PubMed]
- Ogasawara S, Yano H, Higaki K, et al. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res 2001;20:97-113. [Crossref] [PubMed]
- Xu YF, Yang XQ, Lu XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun 2014;446:54-60. [Crossref] [PubMed]
- Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov 2013;3:636-47. [Crossref] [PubMed]
- Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014;19:235-42. [Crossref] [PubMed]
- Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 2014;45:1630-8. [Crossref] [PubMed]
- Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 2014;10:e1004135. [Crossref] [PubMed]
- Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014;59:1427-34. [Crossref] [PubMed]
- Borad MJ, Gores GJ, Roberts LR. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr Opin Gastroenterol 2015;31:264-8. [Crossref] [PubMed]
- Guagnano V, Kauffmann A, Wöhrle S, et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov 2012;2:1118-33. [Crossref] [PubMed]
- Tabernero J, Bahleda R, Dienstmann R, et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors. J Clin Oncol 2015;33:3401-8. [Crossref] [PubMed]
- Kwak Y, Cho H, Hur W, et al. Antitumor Effects and Mechanisms of AZD4547 on FGFR2-Deregulated Endometrial Cancer Cells. Mol Cancer Ther 2015;14:2292-302. [Crossref] [PubMed]
- Nakanishi Y, Akiyama N, Tsukaguchi T, et al. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther 2014;13:2547-58. [Crossref] [PubMed]
- Zhao WM, Wang L, Park H, et al. Monoclonal antibodies to fibroblast growth factor receptor 2 effectively inhibit growth of gastric tumor xenografts. Clin Cancer Res 2010;16:5750-8. [Crossref] [PubMed]
- Feng S, Wang F, Matsubara A, et al. Fibroblast growth factor receptor 2 limits and receptor 1 accelerates tumorigenicity of prostate epithelial cells. Cancer Res 1997;57:5369-78. [PubMed]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005;5:761-72. [Crossref] [PubMed]
- Sang J, Acquaviva J, Friedland JC, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov 2013;3:430-43. [Crossref] [PubMed]
- Jin Y, Zhen Y, Haugsten EM, et al. The driver of malignancy in KG-1a leukemic cells, FGFR1OP2-FGFR1, encodes an HSP90 addicted oncoprotein. Cell Signal 2011;23:1758-66. [Crossref] [PubMed]
- Laederich MB, Degnin CR, Lunstrum GP, et al. Fibroblast growth factor receptor 3 (FGFR3) is a strong heat shock protein 90 (Hsp90) client: implications for therapeutic manipulation. J Biol Chem 2011;286:19597-604. [Crossref] [PubMed]
- Acquaviva J, He S, Zhang C, et al. FGFR3 translocations in bladder cancer: differential sensitivity to HSP90 inhibition based on drug metabolism. Mol Cancer Res 2014;12:1042-54. [Crossref] [PubMed]
- Gudernova I, Vesela I, Balek L, et al. Multikinase activity of fibroblast growth factor receptor (FGFR) inhibitors SU5402, PD173074, AZD1480, AZD4547 and BGJ398 compromises the use of small chemicals targeting FGFR catalytic activity for therapy of short-stature syndromes. Hum Mol Genet 2016;25:9-23. [Crossref] [PubMed]


