Operative microwave ablation for hepatocellular carcinoma: a single center retrospective review of 219 patients
Introduction
Primary cancers of the liver are the 2nd leading cause of cancer related mortality, of which approximately 90% are hepatocellular carcinoma (HCC) (1). Five-year survival rates for all patients diagnosed with HCC is approximately 15%, as disease is frequently complicated by advanced presentation at time of diagnosis and presence of underlying cirrhosis in >80% of patients (1). Orthotopic liver transplantation remains the best treatment option for patients with early disease and cirrhosis, with long-term survival of >70%, whereas in patients with preserved liver function, hepatic resection is the preferred treatment modality (2).
Thermoablative technologies have been widely utilized in the past two decades to treat patients wherein tumor location(s) and underlying liver function must be weighed carefully to prevent significant operative morbidity. Radiofrequency ablation (RFA) is the most widely utilized ablative treatment modality and achieves tumor destruction with low morbidity for lesions smaller than 3 cm (3). However, incomplete ablation and relatively high recurrence rates remain a challenge for RFA technology (4,5). Microwave ablation (MWA) offers several technical advantages to RFA including predictable ablation zone, faster ablative times and non-susceptibility to current and thermal heat sinks within the ablation field (6). Previous multi-institutional analysis of MWA for hepatic lesions (HCC, colorectal and neuroendocrine metastases and other lesions) indicated low morbidity and recurrence rates similar to RFA, with recurrence highest amongst HCC lesions and lesions treated percutaneously (7).
Our institution preferentially performs operative MWA in a minimally invasive fashion for patients with HCC. This allows us to perform intraoperative ultrasound (IOUS) for assessment of the liver and intrahepatic liver lesions, placement of ablation antenna, and real time monitoring of ablation progress and liver lesion treatment. We previously reported on the outcomes from our initial experience with operative MWA reporting on fifty four patients with HCC (8). Over the past 7 years, our institutional experience with this technology and treatment of HCC has grown significantly. We now report on the updated retrospective outcomes from our experience over the past 7 years to evaluate our peri-operative outcomes and long term results of operative, ultrasound (US) guided MWA of HCC.
Methods
Approval for this study was obtained from the Institutional Review Board at Carolinas Medical Center (Charlotte, NC, USA). Retrospective review of medical records and cross section imaging of all patients undergoing operative MWA for HCC between March 2007 and December 2014 was performed. Informed consent was obtained from each patient prior to the procedure. Patient demographics, risk factors for sequelae of liver disease and HCC, as well as pre-operative hepatic function and alpha-fetoprotein (AFP) level were assessed. Cirrhosis was determined by the gross appearance of the liver during operative intervention or by surgical pathology report. Severity of liver disease was determined by the Child-Pugh score and model for end-stage liver disease (MELD) score (9,10). Diagnosis of HCC was established by radiographic appearance on cross sectional imaging using LI-RADS criteria and an elevated AFP level (11). Some patients underwent pre-operative percutaneous biopsy of liver lesions to establish diagnosis prior to referral or underwent intraoperative biopsy prior to MWA. Patients were not required to have tissue diagnosis of HCC to receive MWA treatment. Prior systemic therapy, trans-arterial chemoembolization (TACE), or ablation therapy was recorded. Pre-operative evaluation included cross sectional imaging with either contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) if possible. Tumor information included size, location and number of lesions. Size was recorded as the longest tumor diameter on cross sectional imaging or IOUS. Clinical tumor stage was assigned by American Joint Commission on Cancer (AJCC) 7th edition (12).
Multi-disciplinary evaluation of all patients with HCC is reviewed at our bi-weekly multi-institutional conference. Specialties represented include hepatobiliary surgery, hepatology, interventional radiology, medical oncology, radiation oncology, gastroenterology and transplant surgery with remote providers accessing the meeting via teleconference. Multi-modality therapies are considered for all patients including resection, ablation, transplantation, trans-arterial and systemic therapy. Patients are referred for possible transplant evaluation early on and in general, ablation therapy is considered for patients with both unifocal and multifocal disease as a bridge to transplant particularly if a prolonged wait time is anticipated or if patients may fall outside of transplant criteria due to burden of disease.
Operative MWA technique
All operative MWAs were performed under general anesthesia in a laparoscopic, open or rarely, hand assisted laparoscopic (HAL) technique. Procedures were performed by one of three hepatobiliary surgeons with extensive experience with intraoperative US guidance and MWA (DA Iannitti, JB Martinie, RZ Swan). All operative ablations are guided using intra-operative US (BK Medical A/S, Herlev, Denmark) using either open assisted T shaped probe or laparoscopic probe where appropriate. Ablations were performed using one of two systems: 915 MHz generator with either a 14-gauge surgical antenna or a 13-gauge transcutaneous antenna (Valleylab Inc., Boulder, CO, USA) or a 2.45 GHz generator with either a 5.6 mm diameter operative antenna or a 1.8 mm diameter transcutaneous antenna (AngioDynamics, Latham, NY, USA). The selection of the MWA system used for the procedures was at the discretion of the surgeon however, during the study period, we moved away from using the 915 MHz system and used more exclusively the 2.45 GHz system. The 2.45 GHz system has a higher output with comparatively larger ablation zone achieved over a shorter period of time however as the unit comprises a single generator, serial ablations are generally needed and multiple tumors cannot be ablated at the same time.
Our institutional practice and approach for operative MWA includes creating space between the microwave near field and any adjacent structures such as the gallbladder, colon, duodenum, diaphragm, or heart by mobilization of the liver. Occasionally tagged sponges are inserted into the abdomen to create distance between liver lesions and surrounding structures. Larger tumors (>3 cm) are typically ablated at the deep margin of the tumor first followed by more superficial overlapping ablation zones allowing for continuous US guidance for antenna position and avoidance of obscuring air bubbles within the ablation zone. Intraoperative US is also used to assess color flow Doppler to ensure the tumor is located within the ablation zone. For tumors located in the right posterior sector of the liver, the patient is typically placed in a left decubitus position with the R arm extended over, shoulder in neutral position. The arm is supported by an arm board, legs supported by pillows and the patient is taped and strapped at multiple locations for security.
Operative details assessed in this study included ablation characteristics, tumor location by hepatic segment, type of generator used, and for each tumor the number of applications of ablation, power setting and duration of ablation. In addition, estimated blood loss (EBL), procedure time, and concomitant procedures were recorded.
Postoperative analysis, recurrence
Postoperative outcomes including hospital length of stay (LOS) and readmission within or beyond 30 days following ablation were recorded. Postoperative complications within 30 days following ablation were recorded and stratified according to the Clavien-Dindo classification system (13). Our institutional practice for post ablation imaging follow up includes a three phase liver CT scan performed within 4–6 weeks following surgery. Scans are repeated every 4 months for the first 2 years and then every 6 months thereafter to evaluate for recurrence. In selected patients who either cannot receive IV contrast dye or in whom, liver lesions cannot be visualized by CT scans, MRI of the abdomen is obtained preferentially with Eovist for evaluation of liver lesions and evidence of recurrence following treatment.
Incomplete ablation is defined as enhancement present during the arterial phase imaging at the ablation site on the first post ablation CT scan consistent with residual tumor. Local recurrence is defined as an enhancing lesion contiguous with the ablation site present on subsequent imaging and not identified on the initial post ablation scan. Regional recurrence is defined as intra-hepatic recurrence that is not adjacent to the ablation site. Metastatic recurrence is defined as extra-hepatic recurrence including adrenal, pulmonary and lymph node metastases. Image interpretation for evidence of incomplete ablation or recurrence was determined by the institutional radiologists, the treating surgeon and the investigators for consistency and concordance with assessment and plan. Further liver directed therapy is defined as any additional hepatic ablation therapy performed for incomplete ablation or recurrent disease, post ablation TACE, or radiation therapy. Survival was calculated from the initial ablation procedure until last recorded follow up, liver transplantation or death.
Statistical analysis
Descriptive statistics were used to calculated quantitative data including mean values, standard deviations and proportions and were used to analyze patient and operative outcomes. Univariate analysis was performed using the log-rank test of equality for categorical variables in order to identify clinical variables (Child-Pugh class, MELD score, etiology of cirrhosis), tumor factors (size, multicentricity), and treatment related factors (TACE, complications, recurrence) predicting overall survival. Survival and disease-free survival was estimated using the Kaplan-Meier method. Statistical analysis was performed using Stata (StataCorp, version 13, TX, USA).
Results
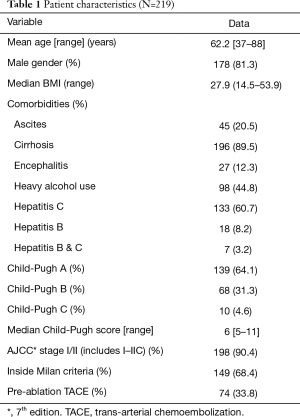
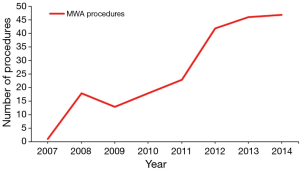
Two hundred and nineteen patients with HCC were treated with operative MWA at our institution during the study period. Table 1 provides patient characteristics, liver dysfunction analysis and comorbidities. Over the study period the number of MWA procedures performed at our institution significantly increased (Figure 1). With the addition of a fourth hepato-pancreato-biliary (HPB) surgeon to the practice in 2012, this number has plateaued between 40–50 procedures a year. The majority of patients were cirrhotic (89.5%), with the most common etiology of cirrhosis being viral hepatitis (hepatitis C 60.7%, hepatitis B 8.2%, both hepatitis B and C 3.2%). The majority of patients were Child-Pugh class A (64.1%) and fell within Milan criteria (68.4%). One third of patients received pre-MWA TACE, done most commonly for lesions >3 cm in size or patients with multiple lesions visualized on pre-operative imaging.
Full table
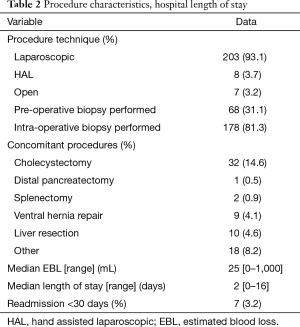
The majority of patients underwent a minimally invasive operative procedure (93.1% were treated purely laparoscopic and 3.7% with hand assisted laparoscopy). Table 2 provides details regarding the procedures and hospital stay. The most commonly performed concomitant procedure was cholecystectomy which is frequently performed if the ablation location is near the gallbladder fossa. Median EBL was 25 mL. One patient had a concomitant liver resection with EBL of 1,000 mL. Median hospital LOS was 2 days (range was up to 16 days for patients with concomitant procedures and some patients were discharged the day of the procedure, LOS =0).
Full table
Table 3 provides details regarding postoperative complications and mortality within 30 days. The most common postoperative complications were urinary retention (10.5%), respiratory insufficiency manifesting as prolonged need to remain in the hospital on oxygen support (8.7%), encephalopathy (4.1%) and ascites (4.1%). Complications occurred in 78 patients postoperatively of which, the vast majority of complications were Clavien grade I/II. Five patients experienced Clavien grade IIIA complications which included two patients with post MWA portal vein-arterial intrahepatic fistula treated with embolization as an outpatient, one patient experienced bleeding esophageal varices treated with EGD and banding. One patient developed a pleural effusion following MWA requiring thoracentesis and one developed pseudogout requiring joint aspiration in the hospital. Four patients experienced Clavien grade IVA complications requiring return to the ICU which included one patient with atrial fibrillation who required vasopressors, a patient who was reoperated for bleeding and then monitored in the ICU, one patient who experienced an NSTEMI requiring cardiac catheterization, and a patient with respiratory insufficiency.
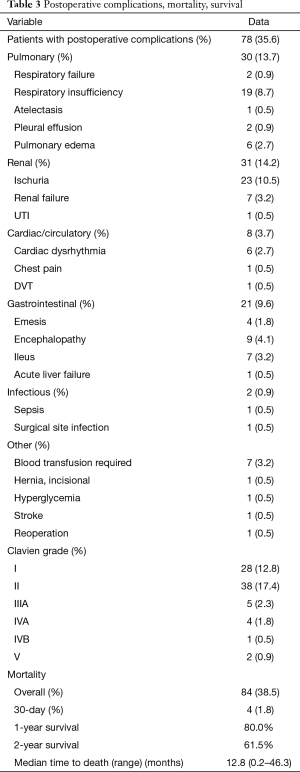
Full table
Survival data were available on 215 patients, four patients left the hospital following ablation and were lost to follow up. Median overall survival was 14.8 months, 1 year survival was 80.0%, 2-year survival was 61.5%. Median time to death was 12.8 months. There were four deaths within 30 days; two patients died following discharge from the hospital after being transitioned to hospice care; one suffered an acute MI and subsequent renal failure, the second had progressive liver dysfunction and was transitioned to home hospice by his primary care provider. Two patients died within 30 days prior to discharge; one patient developed significant post-operative liver failure following MWA and expired in the hospital and another patient suffered a post-operative stroke in the hospital and subsequently died prior to discharge.
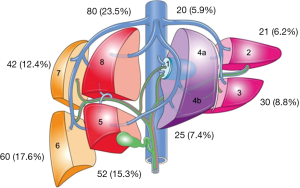
Table 4 provides details regarding the ablations performed for HCC. Three hundred and forty tumors were identified by pre-operative imaging however a total of 348 tumors were ablated following detection with IOUS. The majority of the tumors were located in the right liver (68.8%) with Figure 2 providing a breakdown of the tumor location by liver segment. Fifty-three point five percent of tumors were located in a posterior or subdiaphragmatic location (segments 6 through 8). The median tumor size was 3.2 cm and the median duration of ablation time per patient was 9 minutes. The median number of applications of ablation per tumor was two again with the institutional practice of ablating the deep margin first followed by the more superficial margin. The median duration of application of energy per tumor was 2.6 minutes and the median duration of energy application per ablation was 4 minutes. Total energy applied using MWA was calculated per patient.
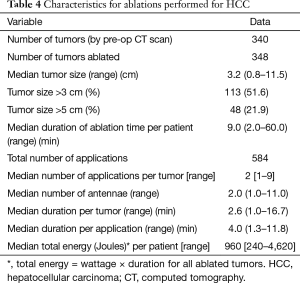
Full table
Table 5 provides a comparison of ablation performed using two different MWA systems, one with 2.45 GHz and the other the 915 MHz. Historically, the 915 MHz system was used more during the beginning of the institutional experience with MWA and then phased out during the more recent years (488 applications for 2.45 GHz vs. 96 applications for the 915 MHz). At the beginning of the study time period, this was the only technology available at our institution. We then added the 2.45 GHz system in 2011. There was no difference in the median size of tumor treated with either of the two systems (P=0.07). The 915 MHz took longer to more effectively ablate liver lesions with duration per tumor and per application both being longer (P<0.01). Median total energy applied per patient was no different with either system.
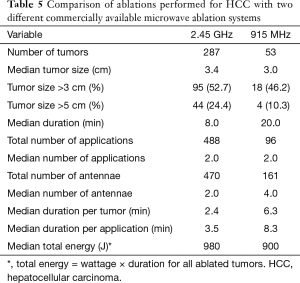
Full table
Post ablation cross-sectional imaging at 6 weeks was available for 89.8% of patients and 81.8% of all tumors (Table 6). Median follow up with imaging was 10.9 months (range, 0–80 months). Incomplete ablation was identified in 2.9% of patients. Almost all patients (95.9%) had post ablation imaging follow up greater than 6 weeks from ablation. Local recurrence at the edge or periphery of the ablation site occurred in 8.5% of tumors at a median of 9.9 months to recur while regional recurrence was seen in 34.8% of patients (21.4% of tumors) at a median of 9.5 months to recur. Metastatic recurrence was seen in 16 patients with a median of 11.8-month time to metastatic disease progression. Overall follow up time with imaging was between 0 months for patients lost to follow up immediately after surgery and up to 80 months. Median overall survival was 14.8 months.
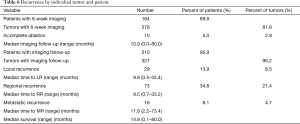
Full table
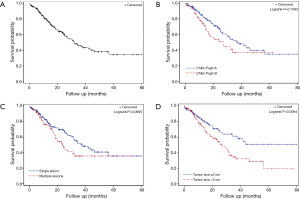
Median 1 year survival was 80% and median 2-year survival was 61.5% (Figure 3A). There was no significant difference in survival between patients who were Child-Pugh class A vs. B (Figure 3B, P=0.11). There was no significant difference in survival in patients who had more than one tumor treated with MWA (Figure 3C, P=0.10). Patients who had a tumor size >3 cm did have a significantly lower survival (Figure 3D, P=0.01).
Discussion
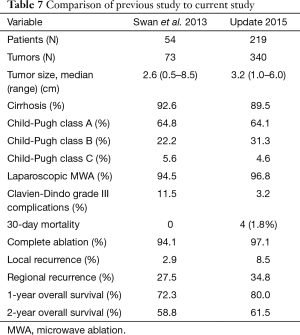
In the current study we present our large, single-center experience of operative US guided MWA for HCC. Patient morbidity decreased in comparison to our previous series, as grade III or higher Clavien-Dindo complications decreased from 11.5% to 3.2% and minimal operative blood loss (Tables 1,7). Operative mortality (<30 days; 1.8%) was consistent with previously reported findings (7). These findings indicate that despite a patient population of mainly cirrhotic individuals, operative MWA can be performed safely. This is underscored by multiple other series again highlighting the safety and effectiveness of operative MWA (14-16).
Full table
Most important from this series, we evaluate our current results in comparison to our previously reported outcomes in 2013 (8). Table 7 provides highlights of the two studies in comparison. Indeed we see an increasing number of patients undergoing laparoscopic MWA (94.5% vs. 96.8%) and increasingly larger size of tumors being treated (2.6 cm median size vs. 3.2 cm). Improved complete ablation rates (at the time of first follow-up imaging) underscore the value of IOUS for effective antenna placement, avoidance of vascular structures and monitoring of ablation efficacy (17). Dedicated use of IOUS also permits detection of additional lesions not observed on preoperative imaging (18). On bivariate analysis of patients who had an incomplete ablation (N=10) versus complete ablation, there were no specific predictive characteristics associated with incomplete ablation. This was irrespective of tumor size (greater than or less than 3 cm). However, because the number of patients with incomplete ablation was quite small, a much larger series would likely be needed to demonstrate significant difference.
While complete ablation rates have continued to improve, meaning we are doing a better job of treating tumors at the time of the operation, we note an increase in the rates of local recurrence (2.9% in the prior study vs. 8.5% in the current evaluation) and regional recurrence (27.8% in prior study and 34.8% in the current study). A component of this could include the increasing size of tumors treated with MWA, which has been shown in other studies to be associated with increased rates of local recurrence (19,20). However, the local recurrence rate remains lower than rates typically reported in the literature for HCC, particularly in comparison to percutaneous MWA (21). Bivariate analysis performed of patients with local recurrence (N=29) versus no local recurrence (N=170) demonstrated that tumor size greater than or less than 3 cm was indeed associated with local recurrence (P=0.02). Median largest tumor size for patients with local recurrence was 3.8 vs. 3.0 cm without local recurrence (P=0.03). Despite an increase in local recurrence rates, median time to local recurrence (9.9 months) was equivalent to median time to regional recurrence (9.5 months), suggesting that local recurrence in the absence of radiographic evidence of incomplete ablation may represent natural disease progression in cirrhotic and/or hepatitis C infected livers.
During the study period, our group transitioned from a 915 MHz system to a 2.45 GHz system. Associated with this change was a reduction in ablation time (20 min, 915 MHz vs. 8 min, 2.45 GHz) and median number of antennae deployed (4.0, 915 MHz vs. 2.0, 2.45 GHz) with a concomitant increase in power deposited per lesion (900 J, 915 MHz vs. 980 J, 2.45 GHz). Although this transition was associated with increased incomplete ablation rates, the median tumor size increased from 3.0 cm with the 915 MHz system to the 2.45 GHz system. A previous study by our group confirmed equivalent efficacy of the two systems with reduced ablation times, a finding supported by theoretical and experimental findings by Curto et al. (6,22).
In response to our findings of increased rates of local recurrence (particularly in patients with larger tumor sizes), we have undertaken a randomized controlled trial to more closely evaluate the role of TACE in patients with HCC less than 3 cm in size, combined with MWA to hopefully provide better local control rates. Although pre-operative TACE had no effect on local recurrence rates (P=0.599), our previous institutional practice included neoadjuvant TACE only for those patients with lesions >3 cm in size or with multiple lesions present. A previous study investigating RFA vs. RFA plus TACE for 2–3 cm HCC lesions demonstrated improved local control and disease-free survival, but equivalent overall survival (23); however, there is a surprising dearth of studies comparing MWA alone versus MWA plus TACE across HCC tumors of all size. We hope that results of this study will continue to guide our institutional practice in determining the most effective way to treat HCC with MWA or multimodal therapy.
Conclusions
Operative MWA with US guidance can be performed safely for the treatment of HCC even in patients with advanced liver disease. The minimally invasive approach affords low morbidity and mortality with acceptable incomplete ablation rates and local recurrence. IOUS is an important component of minimally invasive operative MWA that identifies lesions not observed preoperatively and improves lesion targeting. Further strides can be made to optimize local recurrence prompting institutional practice changes and guided by randomized controlled trials. We hope to continue to evolve our practice patterns for successful treatment of HCC with minimally invasive operative MWA to impact the disease process and improve patient outcomes.
Acknowledgements
The authors would like to acknowledge Megan Templin MS for assistance with statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by institutional review board of Carolinas Medical Center (01-16-23B), and informed consent was obtained from each patient prior to the procedure.
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Azzam AZ. Liver transplantation as a management of hepatocellular carcinoma. World J Hepatol 2015;7:1347-54. [Crossref] [PubMed]
- Feng K, Ma KS. Value of radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Gastroenterol 2014;20:5987-98. [Crossref] [PubMed]
- He ZX, Xiang P, Gong JP, et al. Radiofrequency ablation versus resection for Barcelona clinic liver cancer very early/early stage hepatocellular carcinoma: a systematic review. Ther Clin Risk Manag 2016;12:295-303. [PubMed]
- Yang W, Yan K, Goldberg SN, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 2016;22:2993-3005. [Crossref] [PubMed]
- Simo KA, Tsirline VB, Sindram D, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB (Oxford) 2013;15:991-6. [Crossref] [PubMed]
- Groeschl RT, Pilgrim CH, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 2014;259:1195-200. [Crossref] [PubMed]
- Swan RZ, Sindram D, Martinie JB, et al. Operative microwave ablation for hepatocellular carcinoma: complications, recurrence, and long-term outcomes. J Gastrointest Surg 2013;17:719-29. [Crossref] [PubMed]
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-71. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056-65. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ong SL, Gravante G, Metcalfe MS, et al. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol 2009;21:599-605. [Crossref] [PubMed]
- Cillo U, Noaro G, Vitale A, et al. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford) 2014;16:979-86. [Crossref] [PubMed]
- Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011;104:822-9. [Crossref] [PubMed]
- Donadon M, Torzilli G. Intraoperative ultrasound in patients with hepatocellular carcinoma: from daily practice to future trends. Liver Cancer 2013;2:16-24. [Crossref] [PubMed]
- Takigawa Y, Sugawara Y, Yamamoto J, et al. New lesions detected by intraoperative ultrasound during liver resection for hepatocellular carcinoma. Ultrasound Med Biol 2001;27:151-6. [Crossref] [PubMed]
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299-307. [Crossref] [PubMed]
- Sun AX, Cheng ZL, Wu PP, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol 2015;21:2997-3004. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 2016;32:339-44. [Crossref] [PubMed]
- Curto S, Taj-Eldin M, Fairchild D, et al. Microwave ablation at 915 MHz vs 2.45 GHz: A theoretical and experimental investigation. Med Phys 2015;42:6152-61. [Crossref] [PubMed]
- Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol 2012;81:e189-93. [Crossref] [PubMed]