Phase II study of bevacizumab and preoperative chemoradiation for esophageal adenocarcinoma
Introduction
Esophageal cancer is a relatively uncommon but aggressive malignancy in the United States (1). Several phase III studies have now shown a clear benefit for additional therapy. A standard-of-care is pre-operative chemoradiation for clinical stage III or lymph node-positive esophageal and gastroesophageal junction (GEJ) cancers, based on several phase III trials (2-4).
While 5-fluorouracil (FU) and cisplatin is the reference regimen combined with radiation, the toxicity and cumbersome mode of administration associated with this treatment have led investigators to evaluate more contemporary regimens based on taxanes or irinotecan. Phase II, and, more recently, phase III trials of such regimens indicate comparable outcomes to older regimens (3,5). In particular, the recent Dutch CROSS study utilized carboplatin/paclitaxel with radiation as pre-operative therapy and reported very encouraging results in terms of tolerability and efficacy (3,6).
We previously published the results of a phase II trial of induction and concurrent chemoradiation with cisplatin/irinotecan in 55 eligible patients (7). The results suggested that this regimen was associated with acceptable toxicity but did not appear to be superior to other chemoradiation regimens.
Because of these poor outcomes even with aggressive combined modality therapy, there has been strong interest in the addition of targeted therapies. In esophageal cancer, some studies have shown that elevated baseline levels of vascular endothelial growth factor (VEGF) are associated with worse outcomes following chemoradiation for esophageal tumors (8,9). VEGF expression in tumor tissue has also been noted to increase during chemoradiation (10). Pre-clinical studies have suggested that blockade of VEGF-mediated signaling can potentiate the effect of radiation (11).
Building on the results of our previous experience, we performed a phase II trial of bevacizumab, a monoclonal antibody against VEGF-A, with induction chemotherapy and chemoradiation followed by surgery for patients with locally advanced esophageal and GEJ adenocarcinoma to assess the safety and feasibility of combining this agent with pre-operative chemoradiotherapy. We prospectively evaluated pre-therapy levels of VEGF-A and the use of positron emission tomography (PET) scan after induction chemotherapy prior to chemoradiotherapy.
Methods
This trial was a single-institution, open-label, phase II trial conducted at the Memorial Sloan Kettering Cancer Center (MSKCC). Patients were accrued from September 2006 through April 2011. The protocol was reviewed by the MSKCC Institutional Review Board and patients provided written informed consent. The study was registered with clinicaltrials.gov (NCT00354679).
Eligibility
Patients had resectable endoscopic ultrasound (EUS) staged T2-4Nany or N+ esophageal or GEJ adenocarcinoma, according to the American Joint Committee on Cancer 2002 Staging. GEJ tumors had ≥50% involvement of the esophagus. Patients had a Karnofsky performance status of ≥70% and adequate hematologic (HT), renal, and hepatic function. Exclusion criteria included disease involving celiac or supraclavicular lymph nodes, tumor invasion of the tracheobronchial tree, and/or prior chemotherapy or radiation therapy. Patients with Gilbert syndrome or those taking antiepileptic medications were also excluded.
Treatment
Induction chemotherapy was given on weeks 1 through 5, followed by concurrent chemoradiation on weeks 7 through 12. Patients received cisplatin 30 mg/m2 over 30 minutes, followed by irinotecan 65 mg/m2 over 30 minutes given once a week, on days 1 and 8 every 21 days. For patients ≥60 years old, the irinotecan dose was decreased to 50 mg/m2.
Bevacizumab 7.5 mg/kg was administered every 21 days on weeks 1, 4, 7 and 10 of chemotherapy. The initial dose was administered over 90±15 minutes. If no infusion-related adverse events occurred, the second infusion was then administered over 60±10 minutes. If this was well tolerated, all subsequent infusions were delivered over 30±10 minutes. After two 3-week cycles of chemotherapy/bevacizumab, patients received two 3-week cycles of chemotherapy/bevacizumab administered with concurrent radiation.
Postoperatively, patients received maintenance bevacizumab starting ≥6 weeks or after recovery from surgery, once every 3 weeks for 8 doses.
Absolute neutrophil count ≥1,000/µL, platelets ≥75,000/µL, creatinine <1.8 mg/dL, and ≤grade 1 diarrhea or mucositis were required to continue chemotherapy. Bevacizumab was permanently discontinued and the patients were taken off study in the event of an arterial thromboembolic event, grade ≥2 pulmonary or central nervous system hemorrhage or any grade 4 hemorrhage, congestive heart failure, thrombosis, proteinuria or hypertension or gastrointestinal perforation.
Treatment planning for the external beam radiotherapy was performed using computed tomography (CT) scans with oral and intravenous contrasts for all patients. The gross tumor volume was defined as the region including the primary tumor and any involved lymph nodes identified on contrast-enhanced CT scan, PET scan, or EUS. Contours of the gross tumor volume were expanded by 1.5 cm in the radial dimension and by 4 cm supero-inferiorly to generate the clinical target volume (CTV). The planning target volume (PTV) was made by expanding the clinical target volume another 5 mm radially to allow for daily setup error and motion. All patients were treated to a PTV dose of 50.4 Gy with 3D conformal radiotherapy (3DCRT) or intensity modulated radiotherapy (IMRT) plans designed using the MSKCC in-house treatment-planning system. Treatments were delivered on Varian linear accelerators operating at 6 or 15 MV. A 4- or 5-field technique was used for the 3DCRT and IMRT plans, respectively.
Resection occurred 6 to 8 weeks after chemoradiation. All patients underwent Ivor-Lewis esophagogastrectomy.
Study assessments
Before treatment, patients underwent laboratory evaluation, CT scan of the chest/abdomen, esophagogastroduodenoscopy (EGD), barium esophagram, EUS, and 18F-fluorodeoxyglucose PET (FDG-PET) scan. After induction chemotherapy, patients underwent repeat esophagram and PET scan to rule out disease progression. Following chemoradiation, EGD and CT scan were performed prior to surgery.
Patients were also evaluated every 3 months for the first 2 years, then every 6 months for the next 3 years, then annually. EGD and CT scans of chest/abdomen were repeated every 6 months for 2 years, then annually for 3 years.
Toxicity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Vascular endothelial growth factor-A (VEGF-A) measurement
Patients had serum collected at baseline prior to any therapy. The serum samples were measured for circulating VEGF-A using a commercially available ELISA kit, Human VEGF Duoset (R&D Systems, Minneapolis, MN, USA). The manufacturer’s protocols were followed, and samples were measured in duplicate, with the mean value was used as the final concentration. ELISA plates were read using the Emax Precision Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Standard curves were generated and sample values were determined. Intra-assay and inter-assay validation were also performed.
Study design and statistical analysis
The primary endpoint was to assess the tolerability of bevacizumab, cisplatin/irinotecan with radiation followed by surgery by monitoring grade ≥3 HT and non-hematologic toxicities (NHT) separately. The regimen would be considered tolerable if the HT rate was <72% and the NHT rate was <40%. Grade ≥3 HT and NHT rates for cisplatin/irinotecan and radiation were estimated to be 45% and 15% respectively, based on interim analysis of the phase II study of this regimen at MSKCC.
Based on Simon’s optimal two-stage design, a total of 33 patients would be enrolled. In the first stage, 11 patients would be treated. If ≥8 HTs and/or ≥4 NHTs were observed, the trial would be stopped for excessive toxicity. Otherwise, an additional 22 patients would be enrolled. The combination would be declared tolerable if <20 HTs and/or <9 NHTs were observed out of the 33 patients. Type I and II error rates were approximately 90%.
In addition, the median progression-free survival (PFS) would be estimated to ±10 months. Pathologic complete response (pCR) rates would also be estimated to ±17%. If the primary objective of tolerability was met, evaluation of the PFS and pCR rates would form the basis of recommending this regimen for further study.
Differences in PFS and overall survival (OS) were analyzed using the log-rank test. Differences in proportion were analyzed using Fisher’s exact test. Proportions were estimated using the binomial distributions and exact 95% confidence intervals were provided.
Results
Demographics
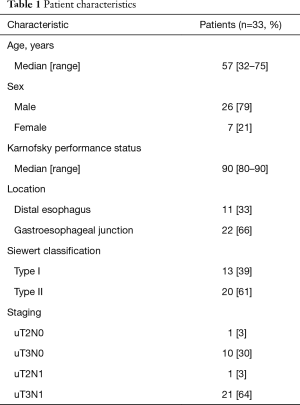
All 33 patients were evaluable, with characteristics summarized in Table 1. The majority of patients (79%) were male. All tumors involved the GEJ; by Siewert classification, 39% and 61% respectively were type I and II lesions. Sixty-seven percent of tumors were uN1+. The vast majority of patients (94%) had T3 tumors.
Full table
Pre-operative therapy delivery and toxicity
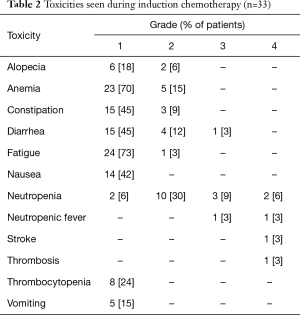
Thirty-two patients (97%) completed induction chemotherapy; one patient developed an embolic stroke after one treatment with chemotherapy and bevacizumab, which was attributed to a patent foramen ovale (Table 2). All of his neurologic deficits subsequently resolved and he was taken off-study.
Full table
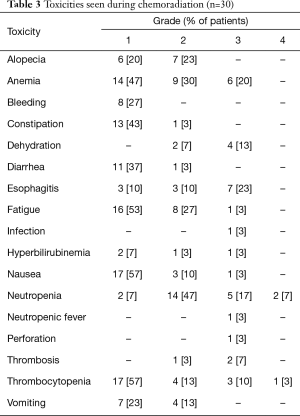
Twenty-eight patients (85%) completed chemoradiation. The four patients who did not complete chemoradiation included two patients with progression or minimal response as assessed by post-induction chemotherapy PET scans, who received alternative chemotherapy with radiation off-protocol. The third patient developed protracted grade 4 HT toxicity requiring discontinuation of chemoradiation. The last patient underwent an endoscopy for grade 3 radiation esophagitis during week 4 of chemoradiation, during which a biopsy was performed and a feeding tube was inserted. Two days later, he developed pneumomediastinum and Staphylococcus bacteremia (Table 3), and he was taken off study. He was treated with antibiotics and subsequently recovered.
Full table
Significant toxicities noted during induction chemotherapy and chemoradiation are shown in Tables 2,3 respectively. Induction chemotherapy was generally well-tolerated but grade 3/4 neutropenic fever occurred in two patients (6%). One patient (3%) developed deep vein thrombosis (DVT) and pulmonary embolism. During chemoradiation, grade 3/4 HT toxicities included neutropenia (7 patients, 23%), anemia (6 patients, 20%), thrombocytopenia (4 patients, 13%) and neutropenic fever (1 patient, 3%). Significant grade 3/4 NHT included esophagitis (7 patients), dehydration (4 patients), DVT (3 patients, 9%). Only one patient (described above) required placement of a feeding tube. Of the 28 patients who had dysphagia at baseline, 27 could be assessed following induction chemotherapy and all (100%) noted improvement or resolution of dysphagia.
Surgery and pathologic response
Of the 28 patients who completed induction chemotherapy and chemoradiation, three patients subsequently developed distant metastases and did not undergo surgery. One patient had a prolonged post-operative recovery and was taken off-study but eventually underwent surgery 6 months after completing chemoradiation. In addition to these 25 patients undergoing surgery (24 on-protocol), the patient with an endoscopy-induced perforation described above underwent delayed resection at 20 months following documentation of locally persistent disease after initially achieving a clinical CR. Therefore, 26 patients (79%) underwent surgery and 25 had R0 resection (76% of the intention-to-treat or ITT population and 96% of operated patienys).
The median length-of-stay following esophagectomy was 10 days (range, 7 to 66 days). Two patients (8%) died post-operatively of respiratory and multi-organ failure, including the patient with delayed recovery post-chemoradiation who was operated on 6 months later. Anastomotic leak was reported in two patients (8%), while four patients developed post-operative atrial fibrillation (15%) and seven patients (27%) were treated for bacterial pneumonia.
Five patients (15% of ITT population; 19% of patients who had surgery) achieved a pCR and another ten patients (30%) had ≥70% treatment response. Of 17 patients with uN1 disease, 8 (47%) had ypN0 disease at surgery; 2 of the 9 patients (22%) with uN0 disease were found to have ypN1 tumors at surgery.
Adjuvant therapy and survival
Twenty-four patients underwent surgery on-protocol and were therefore eligible to receive adjuvant bevacizumab. Of these, four patients did not receive any adjuvant bevacizumab because of an R1 resection, post-operative death, the development of a perirectal abscess or acute femoral artery thrombosis in one patient each. Therefore, 20 patients (83% of those who underwent surgery on-protocol and 61% of the ITT population) received ≥1 dose of adjuvant bevacizumab and 15 patients (63% and 45% of the surgery and ITT population respectively) completed all 8 doses. The five patients who did not complete adjuvant bevacizumab developed recurrent disease.
With a median follow-up of 68 months, the median PFS is 15.1 months and the median OS is 30.5 months. Two- and 5-year survival rates are 64% and 41% respectively. Ten patients (30%) are alive without evidence of disease and three patients (10%) died of other causes (cerebellar astrocytoma in one patient and post-operative deaths in two patients). Of the remaining 20 patients who developed recurrence, 4 developed locoregional recurrence only, 12 developed distant metastases only and 4 had both locoregional and distant recurrence.
Correlation of PET response with therapy outcome
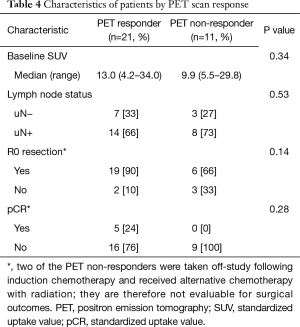
Patients underwent PET scans at baseline and after completing induction chemotherapy (prior to chemoradiation). As the patient who developed an embolic stroke did not undergo a repeat PET scan, 32 patients underwent re-assessment with PET scan. The median standardized uptake value (SUV) in the primary tumor at baseline was 11.5 (range, 4.2–34.0) and after induction chemotherapy was 5.3 (range, 1.5–39.6). We stratified patients into two groups using 35% as the cut point, based on retrospective analysis of our prior study (7) as well as prospective selection of this cut-off by Ott et al. (12). PET responders were defined as SUV decline of ≥35% on the follow-up PET scan compared to the baseline and non-responders as SUV decline of <35%; 21 patients (65%) were PET responders (Table 4). There was not a significant difference in baseline SUV uptake or nodal status in either group.
Full table
We correlated changes in the PET scan after induction chemotherapy with various clinical outcomes, all of which were either statistically superior or show trends toward superiority in PET responders. There was a non-statistically significant trend toward improved R0 resection rates for PET responders vs. PET non-responders (90% vs. 66%, P=0.14). All five patients with pCR were PET-responders while none of the PET non-responders achieved a pCR but this was also not statistically significant (24% vs. 0%, P=0.28). PET responders had superior median PFS (17.0 vs. 6.2 months, P=0.01) and a numerically superior but not statistically significant difference in median OS (51.8 vs. 25.7 months, P=0.4) compared to PET non-responders. Kaplan-Meier curves of PFS and OS as stratified by PET response are shown in Figure 1.

Correlation of baseline serum VEGF-A levels with therapy outcome
Thirty patients (90%) had baseline serum samples that were assayed for VEGF-A level. The median VEGF level was 57.8 pg/mL (range, 1.8–500 pg/mL).
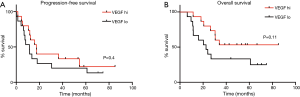
When stratified by the median VEGF-A level, there was no difference in median PFS between patients who were VEGF-Ahivs. VEGF-Alo (10.2 vs. 16.0 months, P=0.4). However, there was a strong trend toward improved median OS for patients who were VEGF-Ahivs. VEGF-Alo (not reached vs. 21.0 months, P=0.11). Kaplan-Meier curves of PFS and OS stratified by baseline VEGF-A levels are shown in Figure 2. Baseline clinical characteristics and other outcomes for VEGF-Ahivs. VEGF-Alo patients are shown in Table 5 and are not significantly different between both groups.

Full table
Conclusions
In this study, the addition of bevacizumab to cisplatin/irinotecan as induction chemotherapy and with radiation did not appear to improve any outcomes. The pCR rate of 15% in this study in the ITT population is identical to the pCR rate in the adenocarcinoma patients on the prior phase II study (7). Similarly, the median PFS and OS of 15.1 and 30.5 months respectively are strikingly similar to the 15.2 and 31.7 months respectively reported previously. A lack of benefit for adding bevacizumab to chemoradiation was also noted in another phase II study by Bendell et al., which added this agent and erlotinib, an oral tyrosine kinase inhibitor against epidermal growth factor receptor, to chemoradiation (13).
The pCR rate of 15% in adenocarcinoma patients in this and our prior phase II study are virtually identical to the pCR rates in the Eastern Cooperative Oncology Group (ECOG) 1201 study, which randomized 90 patients to radiation with either cisplatin/irinotecan (pCR rate 15%) or cisplatin/paclitaxel (pCR rate 16%) prior to surgery (14). Longer-term follow-up also did not reveal a statistically significant difference in median OS for either arm (35 vs. 21 months, respectively), although the median OS for the cisplatin/irinotecan arm is very similar to the median OS in this and our previous study (5). Of note, while these results appear inferior to the pCR rate of 23% and median OS of 43.2 months in adenocarcinoma patients in the phase III Dutch CROSS study (which utilized carboplatin/paclitaxel with radiation) (3,6), ECOG 1201 does provide U.S. data for a platinum/paclitaxel combination with radiation and would suggest comparable outcomes with cisplatin/irinotecan and radiation.
In contrast to the results of this study, two contemporaneous first-line studies at MSKCC that evaluated the addition of bevacizumab to cisplatin/irinotecan (15) and to a modified docetaxel/cisplatin/5-FU regimen (16) in patients with metastatic GEJ and gastric adenocarcinoma suggested significant benefit, compared to historical controls. The phase III AVAGAST study that added bevacizumab to capecitabine/cisplatin chemotherapy in a similar metastatic population failed to demonstrate an improvement in the median OS, although there were improvements in response rates and PFS (17). Subset analysis also suggested a benefit in Pan-American but not Asian patients. More recently, two phase III studies of second-line therapy with ramucirumab, an antibody against VEGF receptor 2, as monotherapy (18) or with paclitaxel (19) revealed improvements in OS, validating the VEGF pathway as a therapeutic target in esophagogastric adenocarcinoma.
The addition of bevacizumab did not increase HT toxicities, when compared to our prior phase II study of this regimen without bevacizumab in 55 patients. For example, grade 3/4 neutropenia during induction therapy was noted in 15% of patients on this study (vs. 31% in the prior study). During chemoradiation, the grade 3/4 neutropenia rate was 23% (vs. 31% previously). On the other hand, NHT may have been increased with the addition of bevacizumab. Grade 3/4 esophagitis during chemoradiation was noted in 23% of patients (vs. 0%), while grade 3/4 dehydration was seen in 13% of patients (vs. 6%). Nevertheless, the feeding tube placement rate remained low and only one patient (3%) required this procedure (vs. 5% in the prior study).
In terms of toxicities of special interest related to bevacizumab, the incidence of venous thromboembolic disease did not appear to be increased. Four patients (12%) developed DVT/PE at some point during pre-operative therapy, compared to eight of 55 patients (15%) on the prior phase II study. One patient did develop an embolic stroke but this was attributed to a patent foramen ovale. Another patient developed a likely esophageal perforation following endoscopic biopsy and feeding tube placement. Any-grade hypertension and proteinuria were not observed in any patients. Surgical complications were not clearly increased with the addition of bevacizumab; the operative mortality rate of 8% in this study (2 of 26 operated patients, including one patient who underwent surgery 6 months after chemoradiation) was similar to the 5% rate noted in our prior phase II study of this regimen without bevacizumab (7). In aggregate, the incidence of anastomotic leak (8% vs. 10%), atrial fibrillation (15% vs. 13%) and pneumonia (27% vs. 18%) also seemed qualitatively similar between the current and previous studies.
While the addition of induction chemotherapy has theoretical benefits, e.g., improvement in dysphagia which may reduce the need for feeding tube placement and the intensification of systemic therapy to treat micrometastatic disease in a moderately chemosensitive cancer, this benefit has not been borne out in any randomized study. In fact, the largest study to evaluate this approach is a randomized phase II study of 126 patients performed by Ajani and colleagues (20). There was no improvement in median OS for patients who received induction chemotherapy (43.7 vs. 45.6 months, P=0.69) and only a non-significant trend toward an improved pCR rate (22% vs. 11%, P=0.094) compared to the chemoradiation-only patients.
Therefore, any potential benefit for induction chemotherapy may arise from the ability to obtain an early assessment of response by PET. In our prior phase II study, we showed that such PET response to induction chemotherapy correlated with improved pCR rate and PFS (7). The results of this study appear to recapitulate our earlier findings, although the smaller numbers of patients on this study likely limited the statistical power. Nevertheless, all five patients who achieved a pCR were PET responders and there was a statistically and clinically meaningful improvement in PFS in the PET responders (from 6 to 17 months).
The fully-accrued Cancer and Leukemia Group B 80803 study (NCT01333033) is evaluating the use of PET scans after induction chemotherapy to guide subsequent chemotherapy during radiation prior to surgery in esophageal and GEJ adenocarcinoma patients. They are randomized to receive induction carboplatin/paclitaxel or FOLFOX (bolus and infusional 5-FU/leucovorin/oxaliplatin) prior to re-assessment by PET. PET-responders (based on the same mSUV cut-off of ≥35%) continue with the same regimen during radiation, while PET non-responders switch over to the alternate regimen with radiation. The primary end-point of the study is to improve the pCR rate in the PET non-responders from a historical 5% to 20%.
In addition, baseline VEGF-A levels also appear to be associated with a trend toward improved OS. Specifically, patients with VEGF-A levels higher than the median measurement had a trend toward improved OS compared to those patients with VEGF-A levels lower than the median (not reached vs. 21 months, P=0.11). Given that all patients received the same treatment, it is not possible to determine from these results if baseline VEGF-A levels may be prognostic or predictive of outcome.
The value of baseline VEGF-A levels as a biomarker was also evaluated in the AVAGAST study (21). Patients with higher VEGF-A levels had worse outcomes if treated with chemotherapy alone, suggesting that it is prognostic in the metastatic setting for patients treated with standard chemotherapy. On the other hand, VEGF-A levels may predict benefit from the addition of bevacizumab: non-Asian patients with higher VEGF-A levels derived benefit from the addition of bevacizumab to chemotherapy (hazard ratio for OS 0.72, 95% confidence interval 0.57 to 0.93). Similarly, our group has evaluated the significance of baseline VEGF-A levels in 147 patients undergoing surgery for locally advanced GEJ and gastric adenocarcinomas (22). Again, higher baseline VEGF-A levels were associated in multivariate analysis with worse OS (5-year OS 49.6% vs. 76%, P=0.009).
Taken together with data from the phase III AVAGAST study in the metastatic setting as well as from our group in the locally advanced setting of gastric and GEJ adenocarcinomas, baseline VEGF-A levels may be useful to stratify or select patients for future trials of anti-VEGF or VEGF receptor therapies in combination with pre-operative chemoradiation.
Acknowledgements
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by MSKCC Institutional Review Board of No. 06-013 and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Kleinberg L, Catalano PJ, Forastiere AA, et al. Long-term survival outcome of E1201: An Eastern Cooperative Oncology Group (ECOG) randomized phase II trial of neoadjuvant preoperative paclitaxel/cisplatin/radiotherapy (RT) or irinotecan/cisplatin/RT in endoscopy with ultrasound (EUS) staged esophageal adenocarcinoma. J Clin Oncol 2012;30:abstr 69.
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Ilson DH, Minsky BD, Ku GY, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer 2012;118:2820-7. [Crossref] [PubMed]
- Imdahl A, Bognar G, Schulte-Mönting J, et al. Predictive factors for response to neoadjuvant therapy in patients with oesophageal cancer. Eur J Cardiothorac Surg 2002;21:657-63. [Crossref] [PubMed]
- Shimada H, Hoshino T, Okazumi S, et al. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous cell carcinoma. Br J Cancer 2002;86:552-7. [Crossref] [PubMed]
- Kulke MH, Odze RD, Mueller JD, et al. Prognostic significance of vascular endothelial growth factor and cyclooxygenase 2 expression in patients receiving preoperative chemoradiation for esophageal cancer. J Thorac Cardiovasc Surg 2004;127:1579-86. [Crossref] [PubMed]
- Kozin SV, Boucher Y, Hicklin DJ, et al. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res 2001;61:39-44. [PubMed]
- Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006;24:4692-8. [Crossref] [PubMed]
- Bendell JC, Meluch A, Peyton J, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol 2012;10:430-7. [PubMed]
- Kleinberg K, Powell ME, Forastiere A, et al. E1201: An Eastern Cooperative Oncology Group (ECOG) randomized phase II trial of neoadjuvant preoperative paclitaxel/cisplatin/RT or irinotecan/cisplatin/RT in endoscopy with ultrasound (EUS) staged adenocarcinoma of the esophagus. J Clin Oncol 2007;25:4533.
- Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006;24:5201-6. [Crossref] [PubMed]
- Shah MA, Jhawer M, Ilson DH, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol 2011;29:868-74. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2013;24:2844-9. [Crossref] [PubMed]
- Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [Crossref] [PubMed]
- Park do J, Yoon C, Thomas N, et al. Prognostic significance of targetable angiogenic and growth factors in patients undergoing resection for gastric and gastroesophageal junction cancers. Ann Surg Oncol 2014;21:1130-7. [Crossref] [PubMed]


