Radioembolization with 90Y glass microspheres for the treatment of unresectable metastatic liver disease from chemotherapy-refractory gastrointestinal cancers: final report of a prospective pilot study
Introduction
The liver is a common site of metastatic involvement by many solid tumors, particularly tumors arising in the gastrointestinal tract such as colorectal, neuroendocrine, and biliary tract cancers. In such patients, liver decompensation contributes to morbidity, and liver failure is often the direct cause of death (1,2). Control of tumor progression in the liver may, therefore, improve patient outcomes even in the setting of extrahepatic disease.
Loco-regional endovascular ablative therapies including selective internal radiation therapy (hereafter referred to as radioembolization) were originally developed for hepatocellular carcinoma (HCC). These forms of treatment are based on the principle that tumors derive their blood supply from the hepatic artery, and tumor perfusion is several-fold higher than perfusion of surrounding liver parenchyma (3,4). Radioembolization involves trans-catheter arterial delivery of 20–60 mm microspheres containing Yttrium-90 (90Y) radioisotope into the tumor microvasculature (5).
Studies documenting safety and efficacy of radioembolization for hepatic metastases from colorectal (CRC) (6-8), neuroendocrine tumors (NET) (9-12), and intrahepatic cholangiocarcinoma (13-17) largely represent retrospective case series. However, there has also been a growing number of prospective studies documenting the use of radioembolization for treatment of metastatic CRC (18-20) and describing the use of 90Y glass microspheres for patients with hepatic metastases from a variety of tumor types (21,22). These studies suggest that radioembolization can be performed safely (18,20-22) and may confer a survival advantage in patients with chemotherapy-refractory mCRC (19).
Prospective data on safety and efficacy of 90Y glass microspheres for patients with metastatic gastrointestinal cancer to the liver remains limited (21,22). Our group previously published preliminary safety and efficacy data from a pilot study that included 30 patients with metastatic chemotherapy-refractory gastrointestinal cancer to the liver (23). The study was subsequently expanded to enroll 12 additional patients. We present here the final report from 42 patients enrolled in a prospective pilot study aimed to determine the feasibility, safety, and efficacy of radioembolization using 90Y glass microspheres for treatment of metastatic, liver-dominant, chemotherapy-refractory gastrointestinal malignancies.
Methods
This Health Insurance Portability and Accountability Act-compliant prospective pilot study was approved by the Committee on Human Research (CHR) of the Institutional Review Board (IRB) at our institution. An Investigational Device Exemption (IDE) application was filed with the United States Food and Drug Administration (FDA; IDE number G090043). The study was registered on ClinicalTrials.gov (identifier NCT01290536). Parameters for patient selection, 90Y glass microsphere dosimetry, treatment, and follow-up for the prospective pilot study have been described in detail previously (23).
Eligibility criteria
Key inclusion criteria were: (I) a diagnosis of progressive metastatic/unresectable malignancy of gastrointestinal origin with liver dominant disease (presence of limited and asymptomatic extrahepatic disease was permitted at discretion of treating investigator and study chair); (II) adequate hepatic laboratory parameters within 30 days of treatment, including alanine and/or aspartate aminotransferase <5 times upper normal limit and serum bilirubin <2 mg/dL; and (III) Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2. Key exclusion criteria were: (I) potential delivery of greater than 30 Gy of radiation to the lungs during a single 90Y glass microsphere administration or cumulative delivery of greater than 50 Gy to the lungs over multiple treatments; (II) evidence of any detectable flow to the stomach or duodenum mapped by Technitium-99m macroaggregated albumin (Tc-99m MAA), despite embolization aimed to stop such flow; (III) previous radiation therapy to the lungs and/or to the upper abdomen; (IV) receipt of chemotherapy within 30 days prior to (or anticipated need within 30 days after) radioembolization, with the exception of concurrent therapy with somatostatin analogues for patients with a NET; or (V) infiltrative tumor appearance on cross-sectional imaging, tumor replacement of >70% of liver volume measured by computed tomography (CT) or magnetic resonance imaging (MRI) volumetry, or tumor replacement of >50% of liver volume with serum albumin level <3 mg/dL.
Patient evaluation
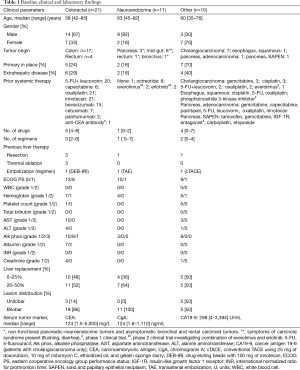
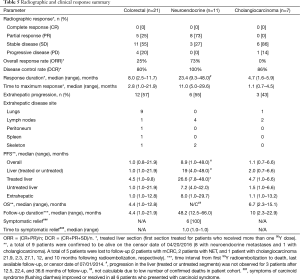
Eligible patients were identified and referred by a medical oncologist (CEA, EKB, RKK, AHK, WMK, KVL, or APV). Hepatic disease progression with liver-dominant growth pattern was confirmed by two consecutive imaging studies (CT or MRI) obtained within 60 days of the first radioembolization. A clinical evaluation including medical history, physical examination, Eastern Cooperative Oncology Group (ECOG) performance status, and laboratory tests [complete blood count (CBC), metabolic and coagulation panels, as well as serum tumor marker (when appropriate)] was obtained within approximately 30 days prior to the first radioembolization treatment. Baseline clinical and laboratory parameters are summarized in Table 1.
Full table
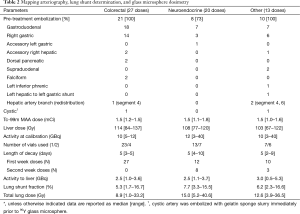
Between 5 and 14 days prior to the first radioembolization, patients underwent a planning visceral arteriography, protective coil embolization of hepatic artery branch vessels, and a lung shunt study using 1.0–1.8 mCi of Tc-99m macroaggregated albumin (MAA) particles. A majority of patients underwent embolization of the gastroduodenal and right gastric arteries or other vessels at risk for non-target radioembolization (24). Vessels targeted for protective embolization and lung shunt study results are summarized in Table 2. Hepatic arterial flow consolidation (25,26) was attempted in three patients by embolizing accessory right or left hepatic artery branches. Lung shunt fraction (LSF) was calculated on the basis of planar images using standard methodology (26). Oral prophylactic therapy using a proton pump inhibitor (PPI) (typically omeprazole) was initiated seven days prior to first planned 90Y administration (27).
Full table
Dosimetry and treatment
Lobar radioembolization was performed for all patients. The liver lobe harboring the highest volume of metastatic disease was treated first. For patients who had previously undergone a partial hepatectomy, a part of the remaining liver was targeted during each treatment (usually 1–2 segments at a time). Dosimetry information is summarized in Table 2. Required 90Y glass microsphere activity (TheraSphere®; BTG International, West Conshohocken, PA) was determined based on the target liver absorbed dose of 90–120 Gy, the volume of the liver tissue to be treated, and LSF using standard methodology (26). For the purpose of dose calculations, residual activity in the treatment vial was assumed to be 1%. Following preliminary analysis of toxicity after treatment of the first ten patients, 90Y activity was limited to less than 4 GBq per administration. As a result, absorbed doses of less than 90 Gy were achieved in ten patients with large right liver lobes (Table 2). Extended shelf-life (second week post-calibration) treatment was used for eight patients with estimated hepatic artery branch flow exceeding 2 mL/s, seven of whom had metastatic neuroendocrine tumors.
90Y glass microspheres were administered using a manufacturer-supplied administration set. Most patients were discharged home 4–6 h after radioembolization. A total of seven high-risk patients were admitted for a planned overnight observation (five patients with symptomatic small bowel carcinoid who required intravenous octreotide infusion intra-procedurally and for 20 h after procedure, and two patients with a history of a Whipple procedure and biliary-enteric anastomosis who were admitted for intravenous antibiotics). After the treatment, patients were instructed to continue taking a PPI for 30 days. Discharge prescriptions also included a broad-spectrum antibiotic for 5 days (amoxicillin, ciprofloxacin or clindamycin), as well as an opiate analgesic (typically hydrocodone with acetaminophen) and an antiemetic (typically prochlorperazine). Fatigue was managed with methylprednisolone Dosepak (Pfizer Inc., New York, NY, USA) in patients without a history of diabetes mellitus. In order to prevent carcinoid crisis, patients with hormonally-active carcinoid were instructed to self-inject short-acting octreotide (typically 100 mg 3 times a day for 2 weeks) and take histamine H1 as well as H2 receptor antagonists (typically diphenhydramine and famotidine) instead of a PPI for 2 weeks after the radioembolization.
Patients with bilobar disease or residual/recurrent disease in the treated liver lobe were allowed to undergo up to four 90Y radioembolizations, each of which was to be performed at intervals of at least five weeks. In order to be eligible for repeat radioembolization, patients had to meet all inclusion and no exclusion criteria prior to each procedure, and demonstrate lack of disease progression in the treated liver lobe by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 on cross-sectional imaging of the abdomen obtained one month following each radioembolization treatment.
Follow-up evaluation
Follow-up clinical evaluations (interval history and physical examination) were performed approximately 14, 30, 60, 90, 120, and 180 days after each radioembolization. The follow-up clock was reset at the time of each subsequent radioembolization procedure. In addition, patients were contacted by telephone approximately 1, 7, and 21 days post-procedure in order to review AE. Liver function tests were assessed weekly for the first month after each 90Y dose administration. A panel of laboratory tests including CBC, metabolic and coagulation panels, and serum tumor marker(s) were obtained approximately 30, 60, 90, 120, and 180 days after each radioembolization. Imaging follow-up with a contrast-enhanced CT or MRI of the abdomen and pelvis as well as a chest CT was obtained at 30, 120, and 180 days post-procedure. Additional anti-tumor therapies were prescribed (when appropriate) at least 30 days after completion of all planned radioembolization treatments. After 6 months following last planned radioembolization, clinical, imaging and laboratory follow-up was dictated by standards of clinical care and was performed at the discretion of the treating medical oncologist. For patients with neuroendocrine tumors, clinical and laboratory AE data were collected for up to 24 months. Data was censored on April 20, 2016.
Criteria for evaluation
Feasibility of 90Y glass microsphere administration was determined by the treating interventional radiologist at the time of radioembolization. Successful administration was defined as the ability to deliver the entire intended 90Y dose to the desired sector of the hepatic arterial circulation.
Safety data were assessed based on post-procedure clinical evaluations, laboratory tests, and imaging studies. AE were graded from 1–5 according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Severe adverse events (SAE) were reported to the IRB, the FDA, and the 90Y glass microsphere manufacturer within 10 days of their occurrence and were followed until resolution.
Treatment efficacy was based on the evaluation of post-treatment cross-sectional imaging studies according to RECIST version 1.1. Efficacy assessments were based on calculating the radiographic response in treated hepatic sectors [complete response (CR); partial response (PR); stable disease (SD); or progressive disease (PD)], overall response rate (ORR, defined as sum of CR and PR), disease control rate (DCR, defined as sum of CR, PR, and SD), response duration, and time to maximum response. Progression free survival (overall, hepatic and extrahepatic) was assessed with respect to treated liver segments, untreated liver segments, and extrahepatic disease. Overall survival (OS) was measured. In patients with hormonally active carcinoid, the rate of symptomatic relief and the time to achievement of symptomatic relief were also assessed (patient self-reporting). Standard definitions of treatment efficacy parameters were used (28).
Statistical analysis
The primary objective of the study was to explore the feasibility and safety profile of 90Y glass microspheres in patients with metastatic disease to the liver. The study was initially designed to enroll 30 patients based on the assumption that fewer than 15% of patients would fail to complete the radioembolization procedure (s) for any reason according to the methods proposed by Carter and Woolson (29,30). However, the IRB allowed the study to remain open to accrual after achievement of its enrollment targets in order to provide patients with liver-dominant metastatic gastrointestinal cancers access to treatment with 90Y glass microspheres. Radioembolization with this agent off-study was prohibited at our institution due to local regulatory constraints, which were lifted in November 2013. The same patient selection, treatment, and follow-up procedures were adhered to for the entire study population.
Data analyses included descriptive reports of the number and characteristics of the patients treated and their clinical and AE experiences. Median, minimum, and maximum values were used to describe dosimetry, laboratory, and time parameters.
Results
Patient characteristics
Between June 2010 and October 2013, 42 adult patients with liver-dominant metastatic gastrointestinal cancer to the liver or intrahepatic cholangiocarcinoma were enrolled. Median age at enrollment was 60 years (range, 35–85 years), and 26 patients (62%) were men (Table 1). An asymptomatic extrahepatic primary tumor was in place for nine of the patients (26%); 12 patients (29%) had asymptomatic low-volume extrahepatic metastatic disease at the time of enrollment.
All patients with mCRC had demonstrated disease progression on and/or intolerance to all approved systemic chemotherapy regimens available at the time of enrollment, including fluoropyrimidines, oxaliplatin, irinotecan, bevacizumab, and epidermal growth factor receptor inhibitors (in patients with KRAS wild-type tumors). Median number of prior anti-cancer drugs in the mCRC cohort was 5 (range, 4–8), and median number of treatment regimens was 3 (range, 2–6). Systemic therapies for patients with other tumor types are summarized in Table 1.
A total of eight patients (19%) received prior liver-directed therapies before study enrollment. Five patients underwent partial hepatectomy with or without concomitant or subsequent radiofrequency ablation. Three patients received trans-catheter therapies; one patient with mCRC underwent chemoembolization using irinotecan-eluting microspheres 2 months prior to enrollment; one patient with metastatic rectal carcinoid was treated with bland transarterial embolization (TAE) 1 year prior to radioembolization; and one patient with cholangiocarcinoma underwent chemoembolization using doxorubicin, mitomycin C, ethiodized oil, and gelatin sponge slurry 2 months prior to radioembolization.
As of the data censor date of April 20 2016, 26 patients (62%) underwent additional anti-cancer therapy following radioembolization. In 21 of these patients, radiographic evidence of intrahepatic (14 patients) or extrahepatic (seven patients) disease progression following radioembolization was documented prior to initiation of additional anti-tumor therapy. Of the 21 patients with mCRC, 9 patients (43%) were treated with systemic chemotherapy (7 patients), percutaneous radiofrequency ablation of previously radioembolized lesions two months following 90Y administration (one patient), or resection of primary tumor in the setting of bowel obstruction (one patient). Of the NET patients, 10 (91%) received subsequent therapy with octreotide (eight patients), everolimus (four patients), capecitabine and temozolomide (four patients), axitinib (one patient), sunitinib (one patient), peptide receptor radionuclide therapy (one patient), bland hepatic artery embolization (two patients), repeat 90Y radioembolization (four patients), and transarterial chemoembolization (one patient) while two patients underwent resection of the small bowel primary tumors. Seven of the patients with cholangiocarcinoma and other tumors (70%) underwent systemic chemotherapy (three patients), chemoembolization using irinotecan-eluting microspheres (two patients), bland hepatic artery embolization (one patient), external beam radiation (one patient), and extended left hepatectomy (one patient).
Baseline laboratory and imaging characteristics
Baseline laboratory findings are summarized in Table 1. Most common laboratory abnormalities at baseline involved alkaline phosphatase elevation (28 patients, 67%), anemia (18 patients, 43%), AST elevation (13 patients, 31%), and hypoalbuminemia (12 patients, 29%). Nearly all baseline laboratory abnormalities were CTCAE grade 1 or 2 in severity. The majority of these abnormalities were observed in the non-NET patient groups (Table 1).
Baseline tumor burden in the liver is shown in Table 1. 19 patients had less than 25% of liver parenchyma replaced by tumor, while 23 patients demonstrated 26–50% replacement of normal liver parenchyma. None of the patients had greater than 50% liver involvement by tumor.
Treatment dosimetry
90Y glass microsphere dosimetry information is summarized in Table 2. A total of 60 administrations of 90Y glass microspheres were performed. Twenty six patients received a single 90Y glass microsphere administration, 14 patients underwent two administrations, and two patients had three administrations. Additional radioembolization was not offered to 19 patients with bilobar disease distribution due to documentation of hepatic or extrahepatic progression within 1 month following first radioembolization (12 patients), intolerable toxicity (two patients), low disease burden in the second liver lobe (two patients), extrahepatic vascular supply in the distribution of the target hepatic artery branch (two patients), flow limiting hepatic artery dissection (one patient), and concern for high cumulative lung radiation dose (one patient).
Median absorbed dose was 109.4 Gy (range, 67–137 Gy). Corresponding median administered total activity was 2.6 GBq (range, 0.5–5.3 GBq). During 17 of 60 treatment sessions two or more 90Y glass microsphere dose vials were used in order to target multiple separate branches of the hepatic artery (13 treatment sessions) or to increase the number of administered microparticles for patients with hypervascular lesions (4 treatment sessions). Extended shelf-life (second week) doses were used during 11 treatment sessions.
Feasibility
Successful delivery of the entire prescribed dose (±5%) of 90Y glass microspheres to the intended hepatic artery branch(es) was achieved in all 60 treatment sessions. One planned lobar 90Y glass microsphere administration could not be performed due to a flow-limiting dissection of the left hepatic artery sustained at the time of microcatheter insertion. Manufacturer-recommended radiation safety precautions were followed, and there were no instances of contamination by radioactive materials.
Safety
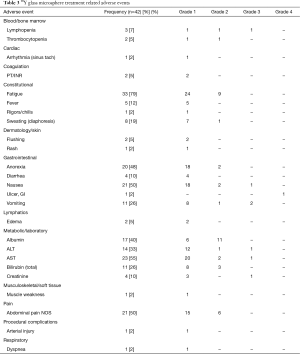
90Y radioembolization was generally well tolerated. The majority of AE were grade 1 or 2 in severity (Table 3). Most common clinical AE were fatigue (33 patients, 79%), nausea (21 patients, 50%), abdominal pain (21 patients, 50%), anorexia (20 patients, 48%), vomiting (11 patients, 26%), sweating (8 patients, 19%), and fever (5 patients, 12%). These AE were transient, and usually resolved within 4 weeks of radioembolization. Common metabolic and laboratory AE included hypoalbuminemia (17 patients, 40%), elevated AST (23 patients, 55%) or ALT (14 patients, 33%), and hyperbilirubinemia (11 patients, 26%). While transaminitis and hyperbilirubinemia usually resolved within 4 weeks of radioembolization, hypoalbuminemia persisted and was associated with functional decline in all affected patients with non-NET primary tumors. A total of nine patients (all with mCRC) developed grade 3–4 hyperbilirubinemia within 1-8 months following radioembolization; however, in every case, this toxicity was attributed to disease progression either with (five patients) or without radiographic biliary obstruction (four patients). One patient with cholangiocarcinoma who underwent extended left hepatectomy six months following left lobe radioembolization developed grade 5 liver failure from inadequate size of the liver remnant and died 2 months after surgery.
Full table
Median calculated LSF was 6.2% (range, 1.7–16.7%), while median cumulative absorbed lung dose was 9.3 Gy (range, 1.0–40.6 Gy). There were no pulmonary AE.
There were five clinically significant AE attributable to administration of 90Y glass microspheres, which included carcinoid crisis requiring unplanned hospital admission 6 days following 90Y radioembolization; two instances of grade 3 vomiting in two patients (each lasting 2 days); and one patient with grade 4 gastric ulcer, which required an antrectomy 3 months following left liver lobe radioembolization. One patient with mCRC who underwent an uneventful administration of 90Y glass microspheres into the right hepatic artery, developed a flow-limiting dissection of the left hepatic artery 6 weeks later during the second planned radioembolization procedure, which prevented safe administration of 90Y glass microspheres. This patient resumed systemic chemotherapy, and did not undergo a second 90Y administration. Details regarding clinical presentation and management of the remaining four clinically significant AE were described previously (23).
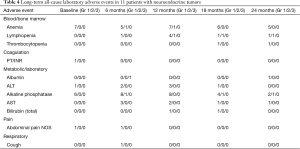
At least 2 years of follow-up safety data was available for 10 of 11 patients with NET. Most long-term treatment-related AE in this group were CTCAE grade 1 or 2 in severity (Table 4). Most common AE were anemia and alkaline phosphatase elevation (typically, grade 1). There were four patients who developed grade 1 lymphopenia, and one patient who developed combined grade 2 lymphopenia as well as grade 1 thrombocytopenia, which persisted 18 and 24 months after radioembolization. This patient received three cycles of peptide receptor radionuclide therapy, totaling 21 GBq of 177Lu and 90Y octreotide over three sessions that started one year following 90Y radioembolization. One patient had transient grade 3 hypoalbuminemia in the setting of malnutrition that resulted from a grade four gastric ulcer.
Full table
Treatment response
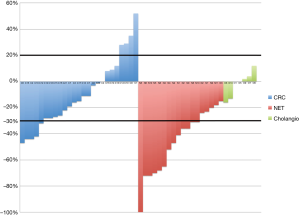
Because of the differences in the magnitude and duration of the response to radioembolization between patients with different primary disease sites, response data are presented for three unique patient groups, mCRC (n=21), NET (n=11), and cholangiocarcinoma (n=7). A summary of radiographic and clinical responses is provided in Table 5. Maximum radiographic responses in the treated liver segments are summarized in a waterfall plot (Figure 1).
Full table
mCRC
In the liver segments that were targeted first, the hepatic ORR was 25%, while hepatic DCR was 80%. Median response duration and time to maximum response in the first treated liver segments was 8.0 and 2.8 months, respectively. Median PFS in the treated liver segments was 4.5 months, whereas in the untreated liver sectors median PFS was 1.0 month. Median overall PFS was 1.0 month (range, 1.0–24 months). Extrahepatic progression developed in 12 patients (57%), most commonly in the lungs. Median extrahepatic PFS was 1.0 months (range, 1.0–12.8 months). Median OS in this cohort was 4.4 months. OS was longer for patients who had PR or SD response to the first radioembolization (median 7.3 months; range, 2.5–12.8 months) compared to patients who had PD in the treated liver lobe (median 3.8 months; range, 1.0–4.4 months; P=0.07). OS was significantly longer for patients who had ECOG PS of 0 at the time of the first radioembolization (median 9.7 months; range, 2.5–12.8 months) compared to patients who had ECOG PS of 1 (median 3.4 months; range 1.0–10.4 months; P=0.03). At least 30% decrease in CEA level from baseline was observed in 3 of 14 patients who had baseline CEA elevation.
The five patients who had more than one 90Y liver lobe treated demonstrated no PRs and one PD event in the second treated liver lobe, and therefore had a lower DCR in the second treated lobe than in the first lobe (Table 6). Response duration in the second lobe and PFS also tended to be shorter.

Full table
mNET
Radiographic responses in the first targeted liver lobe (or segments) in the NET cohort included eight patients with a hepatic PR and three patients with SD, yielding hepatic ORR of 73% and hepatic DCR of 100%. Median time to maximum response was 11.0 months (range, 5.0–29.6 months). After a median of 48.2 months of follow-up (range, 12.5–66.0 months), progression in the treated liver lobe was observed in eight of the patients (73%). The median response duration was 23.4 months (range, 9.3–48.0 months), and median overall PFS was 8.9 months (range, 1.0–48.0 months), whereas PFS for the treated liver sector was 26.6 months (range, 7.8–48.0 months). All but three patients were confirmed to be alive on the data censor date.
All six patients with carcinoid syndrome at the time of the first embolization reported complete or near-complete resolution of hormone mediated symptoms within one month of the first lobar radioembolization treatment. In seven patients with baseline elevation of the serum chromogranin A level, median decrease in the tumor marker level was 69.0% from baseline (range, 5.3–94.4%) at the time of best radiographic response.
Six patients who had more than one liver lobe treated had similar DCRs in both lobes (Table 6). Response duration and time to maximum response were also similar after the first and second radioembolization treatments.
Cholangiocarcinoma
Best radiographic treatment responses to 90Y in seven patients with cholangiocarcinoma included six patients with SD and one patient with PD, yielding a hepatic DCR of 86%. Median response duration and time to maximum response in the first treated liver segments was 4.7 and 1.1 months, respectively. Median PFS in the treated liver segments was 4.7 months, whereas in the untreated liver sectors median PFS was 1.5 months. Median overall PFS was 1.1 months (range, 0.7–6.6 months). Extrahepatic progression in lymph nodes and lungs developed in three patients (43%). Median extrahepatic PFS was 1.1 months (range, 1.0–13.2 months). Median OS in this cohort was 6.7 months (range, 2.3–15.1 months).
Other tumors
The patient with solid and papillary epithelial neoplasm (SAPEN) of the pancreas had hypervascular hepatic lesions at angiography and demonstrated a PR to treatment. Hepatic PFS was 15.8 months, and extrahepatic PFS was 9.2 months (synchronous progression in lungs and lymph nodes was observed). Patients with metastases from esophageal squamous cell carcinoma (n=1) and pancreatic adenocarcinoma (n=1) did not respond to radioembolization.
Discussion
The purpose of this prospective pilot study was to evaluate the feasibility, safety, and efficacy of treatment of liver-dominant metastatic gastrointestinal cancers to the liver using 90Y glass microspheres. At the time of this writing, 90Y glass microspheres are approved only for use under an HDE for treatment of HCC. Because treatment of metastatic disease to the liver is considered an “off-label” use, patients with liver-dominant metastatic gastrointestinal malignancies and cholangiocarcinoma were treated as a part of a prospective pilot study. The evaluation included safety and toxicity as well as efficacy, although efficacy assessment was limited by the heterogeneous nature of the target population.
90Y radioembolization was generally well tolerated. Final analysis of toxicity data demonstrated that the majority of the AE were NCI CTCAE grade 1 in severity. However, the prevalence of AE in this patient population was higher than previously reported in studies of patients with hepatic metastases (21,22) or cholangiocarcinoma (16) who were treated with glass microspheres. For instance, Sato et al. (21) reported on prospectively acquired data for 137 patients treated with 90Y glass microspheres for hepatic metastases from a variety of sources. The most common clinical toxicities reported in that study were fatigue (56%), abdominal pain (26%), nausea (23%), fever (6%), and anorexia (6%). In this study, the prevalence of these side effects was 79%, 50%, 50%, 12%, and 48%, respectively (Table 3). Most common laboratory AEs in this study were AST elevation (55%) and hypoalbuminemia (40%). Unlike in prior studies with 90Y glass microspheres (16,21,22,28), however, there were no events of grade 3–4 hyperbilirubinemia that were attributable to radioembolization.
Clinically significant AE attributable to administration or treatment with 90Y glass microspheres occurred in five patients and included carcinoid crisis, transient grade 3 vomiting, grade 4 gastric ulcer, and flow-limiting hepatic artery dissection that prevented administration of 90Y glass microspheres. These SAE represent expected toxicities from radioembolization. While carcinoid crisis is a recognized complication of TAE (31), this complication has rarely been described after 90Y radioembolization (32). The reported rate of gastrointestinal ulcer formation following radioembolization using 90Y glass microspheres is 0–2% in large series (16,21,22). However, 90Y glass microsphere-induced ulcers may be refractory to medical management (16), as described in this study. Dissection is a recognized complication of hepatic artery catheterization, which has rarely been described in the context of 90Y radioembolization. Patients receiving vascular endothelial growth factor inhibitors (e.g., bevacizumab) are considered to be at an increased risk for this complication (33).
In this study, the cohort of 21 patients with mCRC demonstrated a hepatic ORR of 25% and DCR of 80%, which were similar to the reported efficacy in recently published prospective studies of patients with chemotherapy-refractory mCRC (hepatic ORR of 24% and DCR of 56–86%) (18,19). However, survival results in this study were inferior to those reported in recent prospective studies, which have reported a median PFS ranging from 2.9–4.5 months and OS of 8.8–15.2 months (18,19,21,22). Unlike other studies, which included patients progressing after one systemic therapy regimen, patients in the current study were heavily pretreated and had previously failed all FDA-approved systemic therapies available at the time of enrollment. This might at least in part explain the differences in PFS and OS observed in this study compared to recent literature. Nevertheless, there appeared to be a 3.5-month delay in progression in the treated liver lobe compared to the untreated liver lobe in patients who had only one liver lobe treated. This difference might be in part due to the treatment effect, the magnitude of which is similar to the 3.4-month increase in time to liver progression following 90Y radioembolization using resin microspheres observed in a randomized clinical trial (19). However, the short untreated liver lobe and extrahepatic PFS we observed suggest that 90Y radioembolization may be more beneficial earlier in the course of the disease, in combination with standard chemotherapy, or if used as a “bridge” between two standard chemotherapy regimens.
Patients with cholangiocarcinoma had hepatic DCR of 86%, which was similar to previously reported DCR of 72–98% (14-17). However, median OS of 6.7 months reported in this study was lower than previously described range of 9.3–22 months.
The efficacy outcomes among 11 patients with metastatic NET suggest prolonged hepatic tumor control in this cohort. The radiographic responses (hepatic ORR of 73% and DCR of 100%) appear favorable in comparison to the previously reported rates for ORR (23–64%) and DCR (67–98%) (9-12,34-36). The radiographic responses reported in this study represent best observed responses. This is in contrast to responses observed following a preset 3-6-month time intervals in the other studies, which might explain this discrepancy. In fact, median time to maximum response, which was used to determine ORR and DCR, was approximately 11 months. Median PFS was 8.9 months (range, 4.0–48.0 months), which is comparable to a median PFS of 11.2 months (range, 2.2–27.1 months) previously reported by similar to that reported by Paprottka et al. (36) in a similar cohort. Five patients had sustained hepatic responses that lasted between 23 and 48 months. During 2 years of laboratory follow-up, none of the patients with mNET developed hepatic dysfunction. For patients with liver dominant metastatic carcinoid (a disease that notably lacks approved drugs for systemic therapy), 90Y radioembolization can be used at the time of progression on somatostatin analogue therapy. For patients with pancreatic NET, radioembolization may be helpful as a “bridge” between systemic chemotherapeutic or targeted therapy regimens.
This study has several limitations. Due to its pilot design, this study was not powered to demonstrate efficacy of radioembolization. Patients with liver metastases from a number of primary sites in the gastrointestinal tract were included. The samples of patients in the individual disease subgroups were too small to allow for meaningful conclusions regarding efficacy of radioembolization to be made.
In conclusion, 90Y radioembolization had a favorable safety profile in this prospective study, which was similar to previously published work. The treatment regimen achieved encouraging outcomes, particularly in the mNET cohort.
Acknowledgements
This work was supported by a research grant from Nordion, Inc.
Footnote
Conflicts of Interest: N Fidelman was a recipient of a research grant from Nordion, Inc. (grant term 11/01/2010-10/31/2011), which was used to support this study; the other authors have no conflicts of interest to declare.
Ethical Statement: This Health Insurance Portability and Accountability Act-compliant prospective pilot study was approved by the Committee on Human Research (CHR) of the Institutional Review Board (IRB) at our institution. An Investigational Device Exemption (IDE) application was filed with the United States Food and Drug Administration (FDA; IDE number G090043). Written informed consent was obtained from all patients.
References
- Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11:1057-77. [Crossref] [PubMed]
- Al-asfoor A, Fedorowicz Z. Resection versus no intervention or other surgical interventions for colorectal cancer liver metastases. Available online: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006039.pub3/full
- Llovet JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol 2004;7:431-41. [Crossref] [PubMed]
- Ziessman HA, Thrall JH, Gyves JW, et al. Quantitative hepatic arterial perfusion scintigraphy and starch microspheres in cancer chemotherapy. J Nucl Med 1983;24:871-5. [PubMed]
- Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol 2014;5:178-89. [PubMed]
- Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 2008;19:1187-95. [Crossref] [PubMed]
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 2006;65:412-25. [Crossref] [PubMed]
- Nace GW, Steel JL, Amesur N, et al. Yttrium-90 radioembolization for colorectal cancer liver metastases: a single institution experience. Int J Surg Oncol 2011;2011:571261.
- King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-9. [Crossref] [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. [Crossref] [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [Crossref] [PubMed]
- Devcic Z, Rosenberg J, Braat AJ, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014;55:1404-10. [Crossref] [PubMed]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 2008;113:2119-28. [Crossref] [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [Crossref] [PubMed]
- Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [Crossref] [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [Crossref] [PubMed]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [Crossref] [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [Crossref] [PubMed]
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolizationfor liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [Crossref] [PubMed]
- Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer 2014;13:27-36. [Crossref] [PubMed]
- Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology 2008;247:507-15. [Crossref] [PubMed]
- Benson AB 3rd, Geschwind JF, Mulcahy MF, et al. Radioembolisation for liver metastases: results from a prospective 151 patient multi-institutional phase II study. Eur J Cancer 2013;49:3122-30. [Crossref] [PubMed]
- Fidelman N, Kerlan RK Jr, Hawkins RA, et al. 90Y glass microspheres for the treatment of unresectable metastatic liver disease from chemotherapy-refractory gastrointestinal cancers: a pilot study. J Gastrointest Cancer 2014;45:168-80. [Crossref] [PubMed]
- Lewandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 2007;30:571-92. [Crossref] [PubMed]
- Abdelmaksoud MH, Louie JD, Kothary N, et al. Consolidation of hepatic arterial inflow by embolization of variant hepatic arteries in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol 2011;22:1364-71.e1. [PubMed]
- Spreafico C, Morosi C, Maccauro M, et al. Intrahepatic flow redistribution in patients treated with radioembolization. Cardiovasc Intervent Radiol 2015;38:322-8. [Crossref] [PubMed]
- Naymagon S, Warner RR, Patel K, et al. Gastroduodenal ulceration associated with radioembolization for the treatment of hepatic tumors: an institutional experience and review of the literature. Dig Dis Sci 2010;55:2450-8. [Crossref] [PubMed]
- Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist 2008;13:19-21. [Crossref] [PubMed]
- Carter RE, Woolson RF. Statistical design considerations for pilot studies transitioning therapies from the bench to the bedside. J Transl Med 2004;2:37. [Crossref] [PubMed]
- Winkler RL, Smith JE, Fryback DG, et al. The role of informative priors in zero-numerator problems: Being conservative versus being candid. The American Statistician 2002;56:1-4. [Crossref]
- Lewis MA, Jaramillo S, Roberts L, et al. Hepatic artery embolization for neuroendocrine tumors: postprocedural management and complications. Oncologist 2012;17:725-31. [Crossref] [PubMed]
- Ahuja C, Chadha M, Critchfield JJ. Intraoperative carcinoid hypertensive crisis precipitated by yttrium 90 microsphere radioembolotherapy. Endocr Pract 2010;16:1074-5. [PubMed]
- Brown DB. Hepatic artery dissection in a patient on bevacizumab resulting in pseudoaneurysm formation. Semin Intervent Radiol 2011;28:142-6. [Crossref] [PubMed]
- Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys 2012;83:887-94. [Crossref] [PubMed]
- Cao CQ, Yan TD, Bester L, et al. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg 2010;97:537-43. [Crossref] [PubMed]
- Paprottka PM, Hoffmann RT, Haug A, et al. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol 2012;35:334-42. [Crossref] [PubMed]


