Radiation therapy for hepatobiliary malignancies
Introduction
According to the most recent SEER estimates for 2016, the annual incidence of liver and intrahepatic bile duct cancer is 39,230 cases resulting in 27,170 deaths in the United States, with hepatocellular carcinoma (HCC) accounting for the majority of primary liver cancers (1). In spite of new curative anti-viral regimens with excellent efficacy against chronic hepatitis C virus (HCV) infection, the incidence of HCC appears to be rising domestically by 3.7% in men and 3.0% in women each year (1). Abroad, liver and intrahepatic bile duct cancers account for an even greater percentage of disease and are no less deadly, with HCC ranking as the fourth leading cause of cancer death. HCC has a well-known link with hepatitis B virus (HBV) and HCV as well as heavy alcohol use and aflatoxin B exposure. Familial risk factors include alpha-1 antitrypsin deficiency, Wilson disease, and hemochromatosis. While approximately 43% of hepatobiliary tumors are localized in the liver, relatively small proportions are amenable to surgical resection or transplantation (30% for HCC) with curative intent (2). Furthermore, the availability of donor livers undoubtedly limits the role of curative transplant for those patients who qualify.
Cholangiocarcinoma (CC) ranks as the second most common primary liver malignancy, and represents a deadly and diverse group of cancers, which all originate from bile duct epithelium. Although relatively rare, the incidence can vary widely based on geography from 1.67 per 100,000 in the United States (approximately 2,000 cases annually) to 85.0 per 100,000 in Thailand (3,4). CC is most commonly sporadic but can also be secondary to chronic biliary inflammation and has been associated with primary sclerosing cholangitis, hepatobiliary flukes, hepatolithiasis, HBV/HCV infection, and liver cirrhosis (3,4). CC is broadly classified into two groups: (I) intrahepatic cholangiocarcinoma (IHCC) lesions arising within the liver parenchyma proximal to the second-degree bile ducts; and (II) extrahepatic cholangiocarcinoma (EHCC) lesions arising distal to the second-degree bile ducts. EHCC is further sub-categorized into two groups: (I) hilar or Klatskin CC located within 2 cm of the bifurcation of the left and right hepatic ducts; and (II) distal CC arising from the cystic duct to the Ampulla of Vater and often involving the intrapancreatic portion of the bile duct. The most commonly observed CC locations are hilar (60%) followed by distal (30%) and IHCC (10%). Mucin-producing adenocarcinoma is by far the most commonly observed histologic variant (>90%) (4). Similar to HCC, surgical resection is the only curative modality of treatment for CC.
Patient evaluation
The clinical presentation of CC is frequently associated with symptoms of biliary obstruction including painless jaundice, pruritus, and weight loss, a presentation not dissimilar from that of pancreatic cancer. HCC patients are less likely to present with obstructive symptoms, but jaundice, ascites, and other stigmata of chronic liver disease are common. The social history, with particular attention to risk factors for cirrhosis, such as hepatitis infection and alcoholism, is an important part of the evaluation. Patients with known cirrhosis or chronic HBV infection should be screened for HCC with ultrasound and alpha-fetoprotein (AFP) levels every 6–12 months. Following identification of a lesion, obtaining baseline laboratory values is a valuable initial step in the workup and often includes: CBC, AST/ALT, alkaline phosphatase, bilirubin, ɣ-glutamyltransferase, CEA, CA19-9, and HBV/HCV serologies. In cases of intrahepatic lesions, AFP can be helpful in distinguishing HCC from IHCC. Radiological assessment often begins with an abdominal ultrasound, but should invariably include a triple phase CT scan of the abdomen (arterial, portal-venous, and delayed phase) as well as a CT of the chest and pelvis for complete staging. Though often difficult to distinguish from HCC on contrast enhanced CT or MRI, IHCC usually demonstrates delayed enhancement while HCC more commonly shows rapid arterial enhancement. Lesions with two classic enhancement patterns for HCC do not require pre-operative biopsy, and tissue confirmation is similarly unnecessary if the clinical suspicion for CC is high. Positron emission tomography (PET) can also be of utility in the setting of recurrence following previous liver-directed therapies.
For patients with CC, magnetic resonance cholangiopancreatography (MRCP) is essential in delineating biliary anatomy to help stage these lesions, and may preclude the need for invasive cholangiography. Most patients will undergo endoscopic retrograde cholangiopancreatography (ERCP), which can accomplish three goals: (I) biliary decompression and stent placement in cases of severe obstruction (total bilirubin >10–15 mg/dL); (II) assessment of the aggressiveness and location of the tumor; and (III) assessment of ductal brushings for histologic diagnosis. CC lymph node involvement has been estimated to be as high as 46% in one surgical series (5). For CC, first echelon lymphatic drainage includes hepatic, retroportal, and pancreaticoduodenal stations, while second echelon drainage comprises peripancreatic, aortocaval, and celiac lymph nodes. AJCC 7th edition staging for CC will not be addressed in detail here but does recommend separate staging for intrahepatic, perihilar, and distal subtypes. HCC staging is relatively simple and is based on tumor size, lesion number, and most importantly vascular and organ involvement. Although most patients with HCC present with liver-confined disease, regional lymph node spread to hepatic, hilar, hepatoduodenal, and inferior phrenic lymph nodes is possible.
Standard treatment paradigm
Resectable disease
Hepatobiliary cancer management is determined after stratification into three groups: (I) resectable; (II) unresectable or medically inoperable; and (III) metastatic. The management of any hepatobiliary malignancy requires evaluation by a surgical oncologist and determination of eligibility for curative resection or transplant. The specific criteria for each of these surgeries is beyond the scope of this review, but generally patients with limited HCC and adequate hepatic reserve are eligible for partial hepatectomy, while those meeting United Network for Organ Sharing (UNOS) or Milan criteria should be considered for curative transplantation. Although definitive resection or liver transplantation represent the standard of care for these patients, many patients do not qualify for segmental hepatectomy or transplantation given their disease burden, liver function, or underlying medical comorbidities. Following treatment of HCC with surgical resection or transplantation there is currently no standard recommendation for adjuvant therapy. For patients with chronic HBV/HCV infection, expert consultation with a hepatologist or infectious disease specialist is recommended.
Resectable CC lesions are predominantly treated with surgery with or without neoadjuvant and/or adjuvant therapy. Surgical resection remains the only curative modality of treatment for CC, but unfortunately the majority (50–90%) of patients present with unresectable disease (6,7). The type of surgical resection is dictated by tumor location and is customarily accompanied by regional lymphadenectomy. Generally speaking, distal CC is resected with a pancreaticoduodenectomy (Whipple), IHCC resected with a hepatic lobectomy or liver transplant, and hilar CC resected with a combination of hepatic (commonly caudate) and extrahepatic bile duct resections. Operative mortality for resection at all three tumor locations is generally less than 10% at high volume centers (8). Five-year survival following surgical resection is poorest for hilar CC (10–35%) followed by IHCC (17–40%) and EHCC (23–50%) (8).
Adjuvant therapy for CC
Within the CC resectable cohort, prognosis is dictated by completeness of surgery; unfortunately positive margins occur in up to 85% of patients with hilar tumors (2,9). EHCC is a particularly locally aggressive disease with a predilection for locoregional failure (39–69%) most commonly in the surgical bed and retroperitoneal lymph nodes (10). Despite a high risk for local and distant recurrence, regardless of anatomical subtype, the only category one National Comprehensive Cancer Network (NCCN) recommendation for adjuvant therapy is chemotherapy, typically gemcitabine and cisplatin, following an R2 resection.
After R0 resection without evidence of nodal disease, observation is an appropriate postoperative strategy. Adjuvant chemotherapy (5-FU or gemcitabine-based) can also be recommended and in cases of EHCC adjuvant chemoradiation may be appropriate. In the setting of positive lymph nodes or following an R1 resection there is support for adjuvant 5-FU-based chemoradiation. A meta-analysis of 6,712 biliary tract cancers treated with adjuvant therapy from 1960 to 2010 indicated adjuvant therapy was of greatest benefit in patients with lymph node positive disease or those who underwent an R1 resection (11). Additionally, patients treated with chemotherapy alone or chemoradiation seemed to derive a greater benefit from adjuvant therapy compared to radiotherapy alone, hinting at the importance of distant control in this malignancy.
The majority of evidence for adjuvant chemoradiation comes in the form of small heterogeneous retrospective series, though there is new prospective data supporting its use following surgical resection of EHCC. The recently reported SWOG S0809 was developed to better understand the role of adjuvant therapy following surgery for EHCC (10). Fifty-four patients with CC were enrolled and underwent radical surgical resection. Adjuvant therapy consisted of 4 cycles of combination gemcitabine and capecitabine followed by concurrent capecitabine-based chemoradiation. Radiation was delivered as 45 Gy to the regional lymph nodes with a boost to the preoperative tumor of 54 to 59.4 Gy. Two-year local control was 89% (91% for R0 vs. 84% for R1 resection). An impressive median survival of 34 months was reported and was very similar between those who underwent an R0 and R1 resection. Interestingly, more patients were able to undergo an R0 resection (68%) compared to historical controls. Grade 3 and 4 toxicities were observed in 52% and 11% of patients, respectively, and were primarily hematologic in nature. These encouraging findings will need to be confirmed with future prospective phase III data.
Unresectable HCC
In patients with limited HCC for whom curative surgery is not an option, there is no clear management consensus. According to the 2016 NCCN guidelines, suggested options include ablation [radiofrequency, cryoablation, percutaneous ethanol injection (PEI), or microwave], arterial directed therapies (bland embolization, chemoembolization, or radioembolization), external beam radiation therapy (EBRT), stereotactic body radiation therapy (SBRT), sorafenib, or systemic chemotherapy. None of these guidelines are based on level one clinical evidence, with the exception of systemic sorafenib in Child-Turcotte-Pugh (CTP) Class A patients (12). Currently, there is limited prospective evidence comparing the efficacy of these various forms of liver-directed therapy. Each local treatment carries its own unique set of advantages and disadvantages, and these should be weighed carefully prior to making treatment recommendations. In general, treatment selection for unresectable HCC should be performed in a multidisciplinary setting with input from radiation oncology, surgical oncology, medical oncology, and interventional radiology.
There is evidence to suggest SBRT compared to radiofrequency ablation (RFA) may prove advantageous, particularly for larger tumors. A recent retrospective study of 224 patients from the University of Michigan compared outcomes in patients undergoing either RFA or SBRT. The authors reported that while local control did not vary with tumor size for SBRT, lesions greater than 2 cm in maximal diameter had worse local control when treated with RFA (13). On multivariate analysis, RFA was associated with a hazard ratio of 3.84 (P=0.002) for local progression when compared to SBRT, and overall rates of local progression were higher with RFA. Furthermore, although these treatments were not prospectively evaluated, the authors note RFA was most frequently used for small tumors less than 3–4 cm, while SBRT was typically employed for lesions that were not visible by ultrasound, abutting vascular structures or the luminal gastrointestinal tract, or had previously failed RFA. These results demonstrate excellent SBRT efficacy even in patients with high-risk tumors.
Unresectable CC
The prognosis for locally advanced CC is poor and median survival is often under four months with palliative treatment alone. Death is commonly a result of hepatic dysfunction or cholangitis (14). The low incidence of CC has dramatically limited our ability to enroll for adequately powered prospective trials. As a result, level one evidence for management of unresectable disease is scant with the majority of data garnered from retrospective studies that often include heterogeneous groups of CC locations and non-CC hepatic malignancies. Nevertheless, a 2007 meta-analysis of 1,368 patients with locally advanced biliary tract cancer indicated a superior response rate and tumor control rate were attainable with combination gemcitabine and platinum, which prompted prospective evaluation by the ABC-02 trial (15).
The landmark ABC-02 trial by Valle et al. explored the utility of combination chemotherapy in unresectable and metastatic biliary tract cancer treated with gemcitabine alone versus cisplatin plus gemcitabine (16). Approximately, 25% of patients were determined to have locally advanced disease and nearly 60% had bile duct specific tumors. The addition of cisplatin to gemcitabine was found to yield both a significant progression free (8.0 vs. 5.0 months, P<0.001) and overall survival (OS) (11.7 vs. 8.1 months, P<0.001) benefit. As a consequence, combination chemotherapy remains the standard of care for all anatomical subtypes of unresectable and metastatic of CC. Less evidence exists for the use of chemoradiation or SBRT regimens in the unresectable population, however due to the poor outcomes observed in this cohort proper incorporation of locoregional therapy continues to be investigated.
Historical experience with liver radiotherapy
The initial foray into liver irradiation was beset by the radiobiologic pitfalls of whole liver treatment. Over the last half-century, advances in our understanding of partial- and whole-liver dose tolerances in concert with technological innovation have allowed tumor dose escalation while mitigating damage to healthy liver parenchyma. In the 1970s, the Radiation Therapy Oncology Group (RTOG) conducted a trial of whole liver irradiation in 109 patients with metastatic disease. Of note, the majority of patients had either primary colorectal or lung cancer and only 8% of patients had liver-confined disease. At the time of this trial, localization of tumors in the liver was unsophisticated and in some cases impossible, moreover an inadequate dose regimen of 21 Gy in 7 fractions was utilized (17). In the 1980s, trials were developed for primary HCC specifically to test the viability of this regimen and other hyperfractionated approaches when combined with radiosensitizing chemotherapy (18). Although these techniques were reasonably well tolerated, the efficacy was generally dismal with low doses of radiation, and even with various fractionation schemes and concomitant therapy, local control and survival remained poor (19).
Over time, our ability to identify intrahepatic tumors and deliver more conformal and accurate radiotherapy has dramatically improved. A series of studies at the University of Michigan demonstrated the feasibility of dose escalation using three-dimensional conformal radiation therapy (3D-CRT) with concurrent intra-arterial floxuridine as a radiosensitizer (20,21). In 2005, Ben-Josef et al. reported their experiences using EBRT with BID fractionation and total doses up to 90 Gy for primary HCC, IHCC, and colorectal liver metastasis (22). Of the patients with HCC, at the time of publication only a single patient had experienced progressive local disease. Unfortunately, even with careful CT-based radiation planning and NTCP modeling, the combined rate of grade 3 and 4 toxicity was 30% with one treatment-related death. Furthermore, these results are confounded by concurrent floxuridine usage and BID fractionation, neither of which is generalizable to most radiation oncology practices. Importantly, multivariate analysis of the entire patient cohort including all tumor types, revealed tumor dose to be the most significant predictor of OS, and subgroup analysis found that a tumor dose of at least 75 Gy was associated with improved OS, suggesting a benefit for dose escalated therapy and foreshadowing the utility of high dose per fraction SBRT.
Additional studies of conventionally fractionated EBRT for HCC primarily originate from Asia, with variable results that may reflect demographic differences. The largest reported experience of EBRT for HCC comes from South Korea, where Seong et al. reported a pooled retrospective analysis from 53 hospitals (23). Although there was a wide scope of patient characteristics and treatment techniques, including many patients who received hypofractionated therapy, 81.9% of patients received conventionally fractionated EBRT. Multivariate analysis of data from nearly 400 patients revealed the biologically effective dose (BED) to be a statistically significant predictor of OS. Taken together, the available literature suggests that EBRT for HCC using modern techniques can be an effective tool for local treatment of HCC, with higher doses of radiation associated with better patient survival and local control.
Rationale for SBRT
In recent years SBRT has emerged as an effective treatment option for a variety of malignancies including early stage lung cancer, prostate cancer, and pancreatic cancer (24-26). SBRT represents a novel means of delivering highly conformal ultra-high dose hypofractionated radiation therapy. While studies of conventionally fractionated EBRT for localized hepatobiliary cancers have been mixed, the radiobiological effect of delivering daily doses up to an order of magnitude greater (20 Gy per fraction) than conventionally fractionated EBRT is markedly different. Moreover, the improved ability to reduce high dose to the liver and small bowel with extremely conformal dose distributions allows for dose escalation without undue normal tissue toxicity. Improvement in dose delivery is in part accomplished by precise radiological assessment of tumor and normal tissue location with planning four-dimensional CT, MRI, and PET using half- or whole-body immobilization. Perhaps even more crucial is inter- and intra-fractional motion management especially for those tumors most susceptible to motion located near the diaphragm. Verification of tumor location is commonly performed using fiducial markers placed adjacent to the tumor and corroborated with cone beam CT or kV X-rays obtained prior to each treatment fraction. Intra-fractional motion management can be accomplished with a variety of techniques including abdominal compression, active breath hold, and gating.
For tumor types with lower alpha/beta hypofractionated treatment can result in superior tumoricidal dose and improvements in the radiobiologic therapeutic ratio. A limited number of treatment fractions improves patient access, accessibility, and satisfaction. Furthermore, in an era of escalating health care expenditures there is evidence to support reductions in full course treatment cost with SBRT relative to conventionally fractionated EBRT (27). For patients with metastatic or inoperable hepatobiliary cancer, SBRT may offer the following advantages: (I) the possibility of downstaging with the goal of achieving curative surgery; (II) delay of disease progression while awaiting a suitable donor transplant; (III) minimization of breaks in systemic therapy so crucial in a disease dominated by metastatic failures; and (IV) more durable palliation than conventional EBRT over a much shorter time course. These characteristics make SBRT an appealing treatment modality in the management of inoperable hepatobiliary tumors.
SBRT treatment efficacy
SBRT for HCC
There is a growing body of literature supporting the utilization of SBRT for patients diagnosed with unresectable but localized HCC. In the United States, much of the evidence for liver SBRT lies in the metastatic colorectal patient population. Furthermore, those studies that do include HCC generally also include a heterogeneous group of patients with colorectal cancer metastases and IHCC. The first published report of SBRT for liver tumors including liver primaries and metastatic lesions was published in 1995 in Sweden (28). In this seminal study, Blomgren et al. employed an exhaustive SBRT treatment technique, which utilized a stereotactic body frame, abdominal compression, multiple CT scans in the treatment position, and fluoroscopy of the diaphragm in an attempt to account for tumor movement. A total of 12 primary intrahepatic lesions were treated with a variety of fractionation schemes, including eight patients with HCC and one patient with IHCC. The median survival was reported as 11 months, and complications included fever, sub-capsular hemorrhage, and development or worsening of ascites.
Although more widespread adoption of SBRT for HCC following this publication was initially slow, burgeoning availability of recent technological advances has allowed for more rapid adoption of this promising technique. Nevertheless, the characteristics of a typical HCC patient vary widely across the globe leading to variation in management strategy and patient prognosis. For example, a greater proportion of Japanese HCC patients have resectable disease at presentation compared to their American and European counterparts, likely reflecting differences in competing comorbidities and disease biology (29). Nonetheless, initial reports of SBRT have been quite similar regardless of country of origin. The largest published retrospective analysis of SBRT for HCC alone was reported by Sanuki et al., and consisted of 185 Japanese patients (30). Results demonstrated remarkable improvements since first reports by Blomgren, with 91% of patients achieving local control and 70% of patients alive three years following SBRT. Analogously, North American studies have shown local control rates ranging from 87–100% with OS rates of 67–75% one to two years post-treatment (31-34).
To our knowledge, only six published studies have prospectively evaluated the role of SBRT in patients with HCC and are illustrated in Table 1. The largest of these, published by Bujold et al. in 2013, outlines the pooled analysis from a sequential phase I/II trial undertaken at Princess Margaret Hospital (33). In this report, a total of 102 patients with primary HCC were treated with an individualized six-fraction dose-allocation strategy based on the dose to the liver. All patients had CTP class A liver disease with at least 700 mL of uninvolved liver. Over 60% of patients had multiple liver lesions with a median tumor size of 7.2 cm on cross-sectional imaging. The median gross tumor volume was 117 cc, though there was wide variability in the volumes from 1 to 1,913 cc. The cumulative prescription dose varied from 24 to 54 Gy all delivered in six equal fractions (BED range from 33.6 to 102.6 Gy). One-year local control was excellent at 87% following SBRT, and more than 50% of patients experienced either partial or complete response based on RECIST criteria. The median OS in all patients was 17 months, with 55% and 34% of patients alive at 1 and 2 years, respectively. Additionally, 55% of patients had tumor vascular thrombosis in this study, which was found to be an independent predictor of poor OS on multivariate analysis.
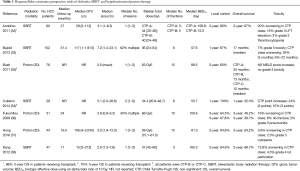
Full table
Treatment with multiple failed liver-directed therapies prior to evaluation for SBRT eligibility is a very common clinical scenario. Of note, in the Princess Margaret study there was marked heterogeneity of prior treatment for enrolled patients. The majority of patients received prior liver-directed therapy most commonly in the form of RFA, TACE, or PEI. A phase II study from Korea, published by Kang et al. in 2012, prospectively evaluated 47 patients with primary HCC who had received 1 to 5 TACE treatments with incomplete response and were considered unsuitable for RFA or PEI (38). Patients on this trial all had CTP class A or B7 disease and received SBRT in three equal fractions to a total dose of 42 to 60 Gy. At 2 years post-SBRT, the local control and OS was 94.6% and 68.7%, respectively, with 38.3% of patients experiencing a complete response by RECIST criteria. Only a single patient (2.1%) was found to have progressive disease at the site of treatment. Despite multiple failed prior TACE procedures, SBRT treatment resulted in a net CTP class decrease from A to B of 10.7%.
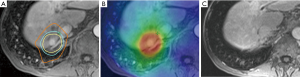
Recurrent HCC treatment with SBRT may prove to be an invaluable arrow in our therapeutic quiver. A retrospective study published by Huang et al. in 2012, examined 36 patients with inoperable recurrent HCC who had previously undergone treatment with curative intent (39). Recurrent lesions were treated to a median dose of 37 Gy in four to five fractions over consecutive days. At 2-year follow up, the local control was 75%, and when compared to a matched-pair cohort that did not receive SBRT there was a statistically significant impact on survival (hazard ratio of 2.39, P=0.009). Taken together these data demonstrate the excellent local efficacy of SBRT in the treatment of primary and recurrent HCC with or without prior liver-directed therapies. Figure 1 illustrates an SBRT treatment plan with follow-up imaging for a patient treated for HCC of segment 7 of the liver.
SBRT for CC
The established efficacy of SBRT in the definitive treatment of upper gastrointestinal tumors prompted extrapolation to the management of unresectable CC. Mahadevan et al. recently reported the results of 32 patients with primarily unresectable IHCC treated with CyberKnife SBRT to a total dose of 30 Gy in 3 fractions with approximately half of these patient receiving adjuvant gemcitabine with or without cisplatin (40). After a median follow-up of 38 months, 1-year local control and OS was 88% and 58%, respectively. The reported median survival of 17 months compares favorably to that seen by Valle et al. of 11.7 months. However, four grade 3 toxicities were reported including: two duodenal ulcers, one liver abscess, and one episode of cholangitis.
Treatment of hilar CC was reported by Kopek et al. and included 27 patients who were treated with SBRT to a higher total dose of 45 Gy in 3 fractions (41). Seventy-eight percent of these patients were deemed inoperable due to surgical unresectability and the remainder due to medical comorbidities or advanced age. Relative to Mahadevan, they reported lower median progression free survival (PFS) and OS of 6.7 and 10.6 months, respectively, possibly reflective of the poorer prognosis generally seen with Klatskin tumors. Nevertheless, one-year local control was excellent at 84% with the majority of patients developing progression outside the treated field. Of note, six patients subsequently developed endoscopically confirmed gastroduodenal ulceration requiring transfusion or hospitalization (25% late toxicity). Duodenum toxicity was clearly dose-dependent with Dmax 1 cc to the duodenum significantly higher for those patients who experienced grade 2 or higher toxicity. It is important to note that in a disease with such a poor survival even with standard treatment, priorities should be placed on minimizing treatment-related toxicity and maintaining quality of life. That being said, the higher toxicity reported by Kopek is largely secondary to the impressively long follow-up (median of 69.6 months). Hence, increased late toxicity may be discernible with longer follow-up using these high dose hypofractionated regimens.
Jung et al. used a similar fractionation scheme in their 2014 report of unresectable and recurrent CC (42). Inclusion was relatively well balanced with only slightly more IHCC relative to EHCC. Median total dose and number of fractions was 45 Gy and 3, respectively, with some patients receiving EBRT prior to SBRT (median 40 Gy in 20 fractions). One-year local control was outstanding at 85% and the median survival was 10 months, despite the majority of treated tumors being recurrent in nature. Relative to the Kopek study, toxicity was less common with six patients reported as having grade 3 or higher late toxicity, three of which had received previous in-field irradiation. Thus, higher BED fractionation schemes may be delivered safely with close attention being paid to adjacent stomach and bowel.
SBRT has been shown to provide excellent local control for unresectable lesions nevertheless out-of-field locoregional and distant failures still drive cancer-specific mortality, thus incorporation of radiotherapy with proper systemic therapy is crucial. Polistina et al. reported a very small cohort with unresectable hilar CC treated with concurrent chemotherapy and SBRT (43). In this study, ten patients were treated to a total dose of 30 Gy in 3 fractions with concurrent gemcitabine (1,000 mg/m2 IV weekly), which was continued following completion of SBRT. After a median follow-up of 35.5 months, there were two reported grade 3 duodenal stenosis events recorded. Three patients were noted to have local progression at one year, though with longer follow-up 60% of patients were noted to progress locally. Concordantly, six patients were seen to have distant failure with a median time to distant failure of 19.5 months. With such a small cohort and such unimpressive results it is hard to draw many conclusions from this study, though it does support the safety of SBRT delivery with concurrent chemotherapy.
SBRT treatment-related toxicity
While the published results of SBRT for HCC have been promising, caution must be employed when irradiating the liver in patients who typically have poor underlying hepatic reserve. Radiation-induced liver disease (RILD), which can present in both classic and non-classic forms, is perhaps the most feared complication of liver irradiation. Although no patients in the Princess Margaret sequential phase I/II trials experienced classic RILD, a decline in CTP class was noted in 29% of patients at three months and death was possibly related to radiation treatment in seven patients. The mean liver dose in patients with suspected treatment-related death was 18.1 Gy (compared to 15.4 Gy, P=0.02). Nonetheless, at 6 months follow-up only 6% of patients demonstrated persistent decrease in their CTP class. Most published series report similar tolerability of SBRT, although increased toxicity has been observed in patients with CTP class C disease (34). Other commonly observed toxicities during treatment are fatigue, anorexia, and nausea, but these are typically mild and frequently do not require intervention (34,42).
The exquisite radiosensitivity of the liver has led to a wide variety of SBRT dosing strategies. NTCP modeling was used to guide dose escalation in the EBRT studies performed at the University of Michigan (21), and these concepts have been applied to SBRT as well. For example, in the Princess Margaret dose escalation trials, adaptive dosing was employed based on an effective volume strategy with a maximum dose of 60 Gy in six fractions. Conversely, other studies have utilized fixed dosing based on CTP class (32). Moreover, given the theoretical increased risk of late toxicity with hypofractionated radiotherapy, careful attention should be paid to luminal GI structures at the time of treatment planning. In the report from Kang et al., a 10.7% combined rate of grade 3/4 GI toxicity was observed (38). Two of these patients (4.3%) experienced gastric ulcer perforation at 7 and 10 months following SBRT completion. Other significant sub-acute and late toxicities that warrant concern include pneumonitis and rib fracture.
SBRT with concomitant sorafenib
The efficacy of sorafenib for primary HCC was first demonstrated with the publication of the SHARP trial in 2008, which provided a 2.8-month median OS benefit compared to placebo (12). However, subsequent phase III trials of surgery, TACE, PEI, or RFA with adjuvant sorafenib failed to demonstrate a similar advantage (44-46). Nonetheless, the survival benefit seen with sorafenib in the initial trial has led investigators to explore its role as a potential radiosensitizer for patients undergoing SBRT. Investigators from Princess Margaret attempted a phase I trial to assess the feasibility of SBRT with concomitant sorafenib (47). Although this was a small concomitant sorafenib dose-escalation trial utilizing a standard 3 plus 3 design, the authors noted significant toxicity. While most patients were able to complete SBRT treatment, sorafenib compliance was poor and only three out of sixteen patients completed their planned regimen without dose modifications. Of grave concern, treatment-related toxicity resulted in two patient deaths (12.5%). While RTOG 1112 remains open to accrual to assess the addition of SBRT to sorafenib in a sequential fashion, there is currently no evidence to support its role in concurrent therapy with SBRT.
Preoperative SBRT prior to liver transplantation
Chief among the difficulties of managing primary HCC in operable patients is finding a suitable donor for liver transplantation. Patients who initially are candidates for curative transplant may suffer from disease progression while waiting for an available donor organ. Liver-directed therapy may have a role in down-staging initially inoperable patients and converting them to candidates for curative transplant. Given the excellent response rates and local control, SBRT provides an intriguing modality as a bridge to transplant and potential conversion of inoperable patients. In 2012, O’Connor et al. reported their long-term experience in ten patients with eleven HCC lesions who received SBRT (median dose 51 Gy in 3 fractions) while awaiting transplantation (48). Patients on this study had a median SBRT to transplant interval of 113 days, with a range of 8 to 794 days, and no patient experienced grade 3 or higher toxicity. At the time of transplant, pathological complete response was noted in 27%. Of the remaining tumors, 75% decreased in size and 25% were stable. With a median follow-up time of 62 months, the overall and disease-free survival were both 100%, suggesting that SBRT may increase the probability of achieving a curative surgery. Additionally, in this series there was no increase in post-operative morbidity compared to institutional controls, suggesting no deleterious effects from preoperative radiotherapy on post-operative wound healing (48).
A similar management strategy for hilar CC was spearheaded at the Mayo Clinic using 5-FU-based neoadjuvant chemoradiation (45 Gy in 1.5 Gy BID fractions) followed by a 20 to 30 Gy intraluminal brachytherapy boost and bridging 5-FU to liver transplant (49). For those who completed transplant, an impressive 5-year OS of 82% was reported. Although these results are promising, there is large a degree of selection bias as all patients underwent exploratory laparotomy and lymph node sampling to confirm localized disease prior to eligibility for transplant. As a result, patients destined for locoregional or distant failure due to subclinical disease or aggressive tumor biology were excluded from analysis.
The University of Michigan is currently exploring a similar method of treatment for unresectable hilar CC with SBRT (50). Twelve patients with unresectable hilar CC were treated with 50–60 Gy in 3–5 fractions followed by maintenance capecitabine until liver transplantation. Following neoadjuvant treatment a total of 35 adverse events occurred with the most common being cholangitis and plantar/palmar erythrodysesthesia secondary to chemotherapy. This elevated rate of cholangitis is likely due to the high doses employed in this study and important to keep in mind when choosing a fractionation schedule for those patients not undergoing liver transplantation. Nine patients were eventually placed on the transplant list with the remaining three disqualified due to laparoscopically identified nodal or metastatic disease. Six patients ultimately underwent liver transplantation yielding a 1-year OS post-transplant of 83%, slightly inferior to the results seen in HCC. Histopathologic analysis demonstrated a partial response in 5 of 6 patients; one patient was found to have a complete response and the remaining partial responders were noted to have only small foci of viable tumor remaining.
Moderately hypofractionated proton therapy
As charged particle therapy becomes more prevalent, its utilization in various disease sites will become ubiquitous. Proton therapy in particular, has clear dosimetric advantages, particularly when compared to the low-dose bath necessary for IMRT, VMAT, or SBRT, although whether this hypothetical advantage provides a clinical benefit for hepatobiliary malignancies is unclear. In 2009, Fukumitsu et al. reported the results of a prospective study of patients with HCC treated with hypofractionated proton therapy (36). Fifty-one patients were treated to 66 GyE in ten fractions. As a group the cohort had largely good underlying liver function with 80% of patients CTP class A. Local control at three and five years was exceptional with rates of 94.5% and 87.8%, respectively. OS was also quite good, with 49.2% and 38.7% of patients living three and five years, respectively. A more recently reported phase II trial of 76 patients from Loma Linda corroborates the potential for proton therapy in a North American population (35). In this study, patients received a definitive dose of 63 GyE in fifteen fractions. Median OS was 36 months, and of the 18 patients who eventually went on to transplant the pathologic complete response rate was 33% and 3-year survival rate was 70%.
The CONSORT study recently reported the results of 92 patients with unresectable HCC or IHCC treated with moderately hypofractionated proton therapy (37). Of note, the tumor size in this study was much larger than previous trials with a median size of approximately 6 cm (median gross tumor volume of 133.7 cc). Median total dose was 58 GyE and all treatments were delivered in 15 equal fractions. After a median follow up of 19.5 months, an outstanding 1-year local control of 97% was observed, which translated into a 22.5-month median OS. Moreover, there was a notably low rate of treatment-related toxicity. Distant metastatic failure was vastly more frequent relative to isolated local failure (53.8% vs. 12.8%). Impressively, only 3.3% of patients demonstrated worsening CTP class, in sharp contrast to the Tse et al. trial where a 23% decline in CTP class within 3 months of SBRT was observed (51). This excellent median survival, amongst the highest reported in the literature, despite inclusion of very large tumors is likely indicative of the superior sparing of normal liver afforded by proton therapy precluding radiation-associated liver toxicity. Proton therapy’s impressive ability to provide outstanding tumor control while minimizing hepatic dysfunction provides a promising management option for inoperable hepatobiliary caner and will be further explored in the phase III NRG GI 001. The definitive experience of cholangiocarcinoma treatment with SBRT and hypofractionated proton therapy is illustrated in Table 2.
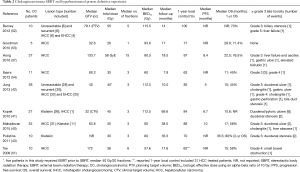
Full table
Conclusions
Hepatobiliary malignancies represent a rare, heterogeneous, and aggressive group of cancers. At present time, cure can only be achieved with surgical resection. Unfortunately in the majority of cases patients are ineligible for surgery. In these situations, multimodality management with a combination of radiation oncology, medical oncology, surgical oncology, and interventional radiology is crucial. Although data is limited, SBRT and proton beam RT can offer excellent local control and appear to improve survival relative to historical data for those patients ineligible for surgical resection. Due to underlying liver dysfunction present in many patients, proton therapy may offer a unique method of hypofractionation and has demonstrated very promising local control in both CC and HCC with minimal toxicity. Nevertheless, distant failure continues to be of primary concern, thus requiring future improvements in systemic therapy.
Acknowledgements
Guy Jensen, MD, contributed to technical review and revisions of the final manuscript. Anatoly Dritschilo, MD, provided general support and guidance for the review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Gunderson LL, Tepper JE, Bogart JA. Clinical radiation oncology. 3rd ed. Philadelphia, PA: Elsevier Saunders, 2012.
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 2013;257:718-25. [Crossref] [PubMed]
- Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128:871-7; discussion 887-9. [Crossref] [PubMed]
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69. [Crossref] [PubMed]
- DeVita VT Jr, Lawrence TS, Rosenberg SA, et al. editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology (Cancer: Principles & Practice (DeVita). Ninth, North American Edition Edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2011.
- Aitken KL, Hawkins MA. The role of radiotherapy and chemoradiation in the management of primary liver tumours. Clin Oncol (R Coll Radiol) 2014;26:569-80. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. [Crossref] [PubMed]
- Park J, Kim MH, Kim KP, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009;3:298-305. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Borgelt BB, Gelber R, Brady LW, et al. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys 1981;7:587-91. [Crossref] [PubMed]
- Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys 1989;17:1223-9. [Crossref] [PubMed]
- Abrams RA, Pajak TF, Haulk TL, et al. Survival results among patients with alpha-fetoprotein-positive, unresectable hepatocellular carcinoma: analysis of three sequential treatments of the RTOG and Johns Hopkins Oncology Center. Cancer J Sci Am 1998;4:178-84. [PubMed]
- Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol 2000;18:2210-8. [Crossref] [PubMed]
- McGinn CJ, Ten Haken RK, Ensminger WD, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol 1998;16:2246-52. [Crossref] [PubMed]
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. [Crossref] [PubMed]
- Seong J, Lee IJ, Shim SJ, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int 2009;29:147-52. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013;87:939-45. [Crossref] [PubMed]
- Gurka MK, Kim C, He AR, et al. Stereotactic Body Radiation Therapy (SBRT) Combined With Chemotherapy for Unresected Pancreatic Adenocarcinoma. Am J Clin Oncol 2017;40:152-7. [Crossref] [PubMed]
- Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 2014;32:1195-201. [Crossref] [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [Crossref] [PubMed]
- Llovet JM. Updated treatment approach to hepatocellular carcinoma. Journal of gastroenterology 2005;40:225-35. [Crossref] [PubMed]
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta oncologica 2014;53:399-404. [Crossref] [PubMed]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [Crossref] [PubMed]
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. [Crossref] [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [Crossref] [PubMed]
- Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol 2014;111:412-7. [Crossref] [PubMed]
- Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053-9. [Crossref] [PubMed]
- Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831-6. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012;118:5424-31. [Crossref] [PubMed]
- Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355-61. [Crossref] [PubMed]
- Mahadevan A, Dagoglu N, Mancias J, et al. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J Cancer 2015;6:1099-104. [Crossref] [PubMed]
- Kopek N, Holt MI, Hansen AT, et al. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol 2010;94:47-52. [Crossref] [PubMed]
- Jung J, Yoon SM, Kim SY, et al. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 2013;8:249. [Crossref] [PubMed]
- Polistina FA, Guglielmi R, Baiocchi C, et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol 2011;99:120-3. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]
- Chen SW, Lin LC, Kuo YC, et al. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;88:1041-7. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Brade AM, Ng S, Brierley J, et al. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2016;94:580-7. [Crossref] [PubMed]
- O'Connor JK, Trotter J, Davis GL, et al. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl 2012;18:949-54. [Crossref] [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458-61. [PubMed]
- Welling TH, Feng M, Wan S, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl 2014;20:81-8. [Crossref] [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [Crossref] [PubMed]
- Barney BM, Olivier KR, Miller RC, et al. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol 2012;7:67. [Crossref] [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [Crossref] [PubMed]
- Ibarra RA, Rojas D, Snyder L, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol 2012;51:575-83. [Crossref] [PubMed]


