Colorectal cancer anatomic distribution patterns remain the same after sessile serrated adenoma/polyp considered cancer precursor: a 9-year comparison study from community-based endoscopy centers
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed in men and women, and it is the third leading cause of cancer death in the U.S. (1). Over the years, efforts to reduce CRC incidence have included food and lifestyle modifications, living standard improvement, and implementation of colonoscopy screening and surveillance programs. These efforts have accounted for the steady decline in the age-adjusted incidence of distal CRCs (2). Unfortunately, the incidence and mortality rates from right-sided CRC have not decreased as expected when compared with the significant reduction in CRCs arising in the left-sided colon (2-4).
Growing evidence indicates that many right-sided and interval CRCs have different molecular profiles than CRC arising in the left-sided colon. Many right-sided CRCs are associated with BRAF mutation, epigenetic alterations such as hypermethylation [CpG island methylator phenotype (CIMP)] and microsatellite instability (MSI). These molecular changes are part of the so-called alternative pathway or serrated pathway (SP) toward colorectal neoplasia. Therefore, it is likely that a proportion of the right-sided and interval CRCs arise from previously undetected SSA/Ps on the initial colonoscopy screening or as a result of incomplete resection of SSA/Ps (5-8).
SSA/P is a relatively new entity that was recognized and accepted in the pathology community as part of the diagnostic terminology in 2003 (9). Routine diagnosis of SSA/P among all pathologists, however, was adopted more slowly till 2005. In 2012, the American Gastroenterology Association (AGA) updated its guidelines to include the management of SSA/Ps (10). It is now estimated that up to 30% of CRCs arise from the SP and approximately 15% of SSA/Ps patients will develop subsequent CRCs or adenoma with high-grade dysplasia (10-12). Therefore, this represents a major challenge to CRC prevention in the general population.
This pilot study was designed to determine whether there have been changes in the anatomic distribution patterns of CRC related to the recognition and diagnosis of SSA/Ps in this large sample. We examined and compared the primary CRCs coming from the general U.S. population between January through December 2014 (SSA/P had been recognized and considered a CRC precursor) and August 2000 through December 2005 (SSA/P was unrecognized as a CRC precursor).
Methods
Study design and patients
This retrospective study was conducted at Miraca Life Sciences (MLS) Research Institute and approved by the Miraca Institutional Review Board (IRB). MLS is a specialized pathology laboratory that includes a large subspecialty gastrointestinal (GI) pathology practice. It receives specimens primarily from community-based ambulatory endoscopy and surgery centers throughout the U.S.
The pathology database at MLS was queried to identify all patients who were given a diagnosis of primary CRC between 2000 to 2005 and in 2014. A CRC diagnosis was established using standard histopathologic criteria by board-certified pathologists in the same institution (MLS). The diagnosis for CRC arising in SSA/P [serrated carcinomas (SCAs)] required the presence of both components of carcinoma and SSA/Ps in the same tissue fragment. All specimens were endoscopic biopsies, fixed in 10% buffered formalin and embedded in paraffin prior to sectioning. Slides were prepared with 4 µ thick sections and stained with H&E.
The patients were divided into two groups: (I) the control group was composed of CRC patients diagnosed from 8/3/2000 to 12/31/2005, a time prior to the acceptance and management of SSA/Ps as a CRC precursor and a time when the same polyp was considered a benign hyperplastic polyp; and (II) the current group was composed of CRC patients diagnosed from 1/1/2014 to 12/31/2014, 9 years after the time when SSA/P became an accepted diagnostic entity and clinically managed like a conventional adenoma as a CRC precursor.
Patients’ demographics, clinical data, endoscopic findings, specimen subsites, and corresponding pathologic findings were all included. Patients with metastasis to the colon from other organs, patients with a history of CRC or with recurrent/residual CRC were all excluded from the study.
Study definition of anatomic sites in the colon
In this study, anatomic subsites were based on their original specimen requisitions submitted by endoscopists, except in 7.5% of patients where the specimens were labeled with the distance from the anal verge, of which there were 9.8% from descending, 63.3% from sigmoid, 11.0% from recto-sigmoid, 14.3% from rectum, and only 1.7% from the transverse colon. Specimens that failed to provide clear documentation of specimen subsites or those without any specific designation were identified as “not otherwise specified (NOS)”.
The following terms were used in the study to define the CRC anatomic distributions: (I) proximal colon CRCs consisted of tumors from the ileocecal valve, cecum and ascending colon; (II) right-sided colon CRCs, tumors from the ileocecal valve, cecum, ascending colon (i.e., proximal CRCs), hepatic flexure, transverse colon, and specimen originally labeled “right”; (III) distal colon CRCs consisted of tumors from the sigmoid colon, recto-sigmoid junction, and rectum; (IV) left-sided colon CRCs, tumors from splenic flexure, descending colon, sigmoid colon, recto-sigmoid junction, rectum (i.e., distal CRCs), and specimen originally labeled “left”.
Statistical analysis
All quantitative data were summarized by mean and standard deviation (SD), “mean ± SD” and count data were given their corresponding percentage “%”. The collected quantitative and count data were then sub-grouped and analyzed using Student’s t-test, Mann-Whitney U test, Chi-square test (χ2) or Fisher’s exact test. P value less than or equal to 0.05 was considered to be statistically significant. All statistical analyses were performed using Sigma Stat v3.5 software (Richmond, California, USA).
Results
A total of 5,685 CRCs in 5,602 patients were identified in the specified time ranges. There were 2,728 patients (65.6±12.7 years, 21–96 years) with 2,765 CRCs in the current group [2014], and 2,874 patients (67.3±13.0 years, 17–96 years) with 2,920 CRCs in the control group [2000–2005]. The mean age of the patients in the current group was 1.7 years younger than the control group (67.3 vs. 65.6 years, respectively, P≤0.001) in Table 1. The ratio of men to women between the current and control groups was essentially equal at 1.2. Overall, the current group of patients showed no statistical difference with regards to CRC anatomic distribution in the proximal, right-sided, distal, and left-sided colon compared with the control group (all, P>0.05). There were statistically significant differences in the number of CRCs arising at the hepatic flexure (4% in current group vs. 5.3% in control group, P=0.032) and at the recto-sigmoid junction (6.9% in current group vs. 3.8% in control group, P≤0.001) in Table 2 and Figure 1. There were also no statistically significant differences in the CRC anatomic distribution pattern by gender in the current and control groups (all, P>0.05) in Table 3.
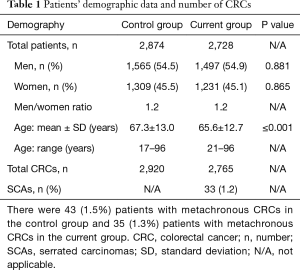
Full table
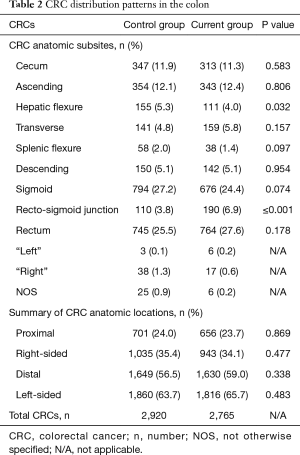
Full table
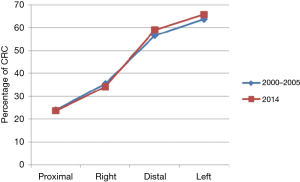
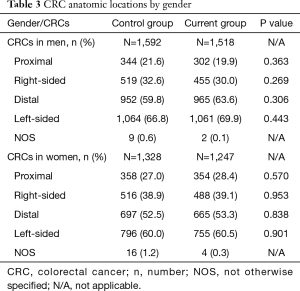
Full table
There were 33 (1.2%) patients with 38 (1.4%) CRCs arising in SSA/P identified in the current group. Among them, there were more women than men with SCAs (69.7% vs. 30.3%). Women were significantly older than men at the time of diagnosis (76.4 vs. 69.6, P=0.023). The SCA anatomic distribution patterns showed a significant right-sided predominance (86.8%) with 52.6% in the proximal colon, shown in Table 4.
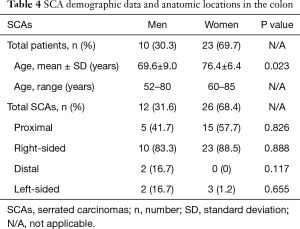
Full table
Discussion
To our best knowledge, this is the first study to follow the consequences of SSA/P diagnosis and possible resultant changes in current CRC anatomic distribution patterns, especially in the right-sided colon, by examining a large population of CRC patients from community-based endoscopy centers nationwide. This study specifically uses detailed anatomic CRC subsites to compare changes in frequency of CRCs in each anatomic subsite before and after SSA/P was considered and managed as CRC precursors. It has been reported that the tip location of endoscopy is fairly accurate (85%) to indicate the anatomic location of the colon (13). Therefore, the accuracy of anatomic subsites we used here should be considered valid in the study.
Our data showed that there were no statistical differences in the overall CRC anatomic distribution patterns between the current and control groups. Exceptions were the hepatic flexure and recto-sigmoid junction areas which were probably due to the inaccuracy of endoscopic orientation of the CRC anatomic subsites by either over or underestimation in these small areas. However, the importance of this study is that there was no decrease in the frequency of right-sided CRCs as might be expected with improved recognition and management of predominantly right-sided SSA/Ps.
These findings should be considered in the context of the study limitations since this is a retrospective study: (I) patients’ nationality and ethnic groups were not known. It has been well noted that the prevalence of SSA/P is about 1.7% to 8.1% in the Western population and less common in Africans, Chinese, and Hispanics compared to Caucasians (14,15). The variation in SSA/P incidence might be influenced by different genetic imprints, lifestyle modifications and dietary factors. We do not know the percentage or composition of each nationality or ethnic group in this study. In addition, clinical information such as patients’ socioeconomic status, smoking history and body mass index (BMI) were not collected. These factors are variably shown to affect CRC biological and histopathological behavior (16,17); (II) a critical factor that may influence our findings is endoscopists’ skills and adenoma detection rates; it has been reported that the adenoma detection rate varies significantly among individual endoscopists from 13.5% to 36.4% (P<0.001), HPs from 7.7% to 31% (P<0.001), and SSA/Ps from 0.3% to 2.2% (P=0.02), and even in the gender of men with 25% and women with 15% variation in adenoma detection rate (18,19); (III) in addition to the varied detection rate from endoscopists, patients with SSA/Ps may be under-diagnosed by pathologists, too. Studies have also shown that pathologists have only moderate inter-observer agreement with the diagnosis of SSA/Ps (20-22); (IV) a time lag exists between the knowledge and action of endoscopists and pathologists regarding SSA/Ps as a CRC precursor in clinical management.
The natural progression of SSA/P to high-grade cytologic dysplasia or CRC is still not well known. It has been reported that when SSA/P develops cytological dysplasia, the malignant transformation rate is faster than the classic adenoma-carcinoma sequence (23). Oono et al. reported that rapid malignant transformation via the SP could be in as short as 8 months (24). Lazarus et al. reported that SSA/Ps have higher recurrence and faster growth rate as compared to conventional adenomas (25). Two separate large cross-sectional studies performed at MLS, however, showed that SSA/Ps may have a more prolonged course to transformation. In these studies, it was estimated to take 9 to 15 years to develop cytologic high-grade dysplasia (2%) or cancer (1%) in SSA/P (26,27). Therefore, the interval of 9 years since the acceptance of the diagnosis of SSA/P used in the current study was felt to be sufficient for early detection of a potential change in the frequency of CRC anatomic distribution.
In this study, 33 (1.2%) patients were identified with CRC arising in a histologically identifiable preexisting SSA/P. Most of these patients (69.7%) were elderly women (76.4 years) in the right-sided colon (88.5%). This percentage is similar to the recent publication from Beg et al., who reported only 0.8% of Middle Eastern CRC cases arising via the SP, but lower than the data from Mäkinen et al., who reported that 5.8% of CRCs arose from SSA/Ps (28,29) and recently Erichsen et al. reported by using a Danish database that the 10-year risk for CRC was 4.4% for patients with SSA/P with dysplasia (30). The reasons for these differences are unclear, but different molecular genetics from different ethnic groups, specimen origins (i.e., biopsy or surgical specimen) (27), follow-up duration and even definition of a SP-associated neoplasm (31-33) might all play roles.
One could assume that if effectively removing SSA/Ps, the actual number of SSA/Ps transforming to CRCs will be decreased, and then one would expect the frequency of CRCs arising in SSA/Ps to be low. However, the frequency of CRCs occurring in each anatomic subsite, especially in the right-sided colon, was essentially the same as 9 years ago in this study, a finding that does not support this assumption. Therefore, other reasons including the detection rate of SSA/Ps, since these lesions are often flat and hidden under mucous caps which increase the difficulty in detecting SSA/Ps by endoscopists, how the diagnosis of SSA/Ps was followed by the clinicians, or 9 years interval might not be long enough to account for a change in the SSA/Ps-inducing CRC (SCA) occurrence. All of these might be the causes associated with failure to impact the frequency of right-sided CRCs. Therefore, further continuous follow-up study is needed to provide a more practicable guideline for the clinical management of SSA/Ps, especially regarding follow-up endoscopy intervals.
Recently, Chino et al. reported direct evidence of CRC arising from a recurrence of SSA/P resected endoscopically 5 years ago (34). Similar findings were also reported by Teriaky et al. and Lu et al. who found that 5% and 12.5% of patients with SSA/P develop CRC within 5 and 21 years follow-up, respectively (35,36). These publications demonstrate that residual SSA/Ps developing into CRCs in a relatively common clinical scenario, suggesting that SSA/Ps not only require early discovery, correct diagnosis, and complete removal (polypectomy), but also require close follow up to be sure the polyp has been completely excised. Therefore, it is suggested that clinical management of SSA/Ps, including a follow-up colonoscopy after endoscopic resection of SSA/Ps, might need a different strategy in the future (37,38).
Conclusions
In conclusion, the current CRC anatomic distribution patterns remain the same compared with 9 years ago, despite SSA/P having been accepted and treated as a CRC precursor. The reasons for these findings remain unclear but SSA/Ps detection rate, residual SSA/Ps, and a follow-up schedule after polypectomy should be considered and further investigation is needed.
Acknowledgements
The authors wish to acknowledge the valuable administrative support of Suzanne Ridner, BS.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Miraca Life Sciences Research Institute IRB (2014-009).
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Cheng L, Eng C, Nieman LZ, et al. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol 2011;34:573-80. [Crossref] [PubMed]
- Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1-8. [Crossref] [PubMed]
- Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst 2010;102:89-95. [Crossref] [PubMed]
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006;131:1700-5. [Crossref] [PubMed]
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010;105:1189-95. [Crossref] [PubMed]
- Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology 2014;146:950-60. [Crossref] [PubMed]
- Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74-80.e1. [Crossref] [PubMed]
- Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003;27:65-81. [Crossref] [PubMed]
- Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29; quiz 1314, 1330.
- Fu X, Qiu Y, Zhang Y. Screening, management and surveillance for the sessile serrated adenomas/polyps. Int J Clin Exp Pathol 2014;7:1275-85. [PubMed]
- Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol 2011;106:229-40. [Crossref] [PubMed]
- Shah SG, Saunders BP, Brooker JC, et al. Magnetic imaging of colonoscopy: an audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc 2000;52:1-8. [Crossref] [PubMed]
- Kumbhari V, Behary J, Hui JM. Prevalence of adenomas and sessile serrated adenomas in Chinese compared with Caucasians. J Gastroenterol Hepatol 2013;28:608-12. [Crossref] [PubMed]
- Leung WK, Tang V, Lui PC. Detection rates of proximal or large serrated polyps in Chinese patients undergoing screening colonoscopy. J Dig Dis 2012;13:466-71. [Crossref] [PubMed]
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009;18:2310-7. [Crossref] [PubMed]
- Kim KM, Lee EJ, Ha S, et al. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol 2011;35:1274-86. [Crossref] [PubMed]
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010;105:2656-64. [Crossref] [PubMed]
- Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc 2012;75:515-20. [Crossref] [PubMed]
- Farris AB, Misdraji J, Srivastava A, et al. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol 2008;32:30-5. [Crossref] [PubMed]
- Khalid O, Radaideh S, Cummings OW, et al. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J Gastroenterol 2009;15:3767-70. [Crossref] [PubMed]
- Ensari A, Bilezikçi B, Carneiro F, et al. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch 2012;461:495-504. [Crossref] [PubMed]
- Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol 2006;125:132-45. [Crossref] [PubMed]
- Oono Y, Fu K, Nakamura H, et al. Progression of a sessile serrated adenoma to an early invasive cancer within 8 months. Dig Dis Sci 2009;54:906-9. [Crossref] [PubMed]
- Lazarus R, Junttila OE, Karttunen TJ, et al. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol 2005;123:349-59. [Crossref] [PubMed]
- Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010;63:681-6. [Crossref] [PubMed]
- Yang JF, Tang SJ, Lash RH, et al. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch Pathol Lab Med 2015;139:388-93. [Crossref] [PubMed]
- Beg S, Siraj AK, Prabhakaran S, et al. Molecular markers and pathway analysis of colorectal carcinoma in the Middle East. Cancer 2015;121:3799-808. [Crossref] [PubMed]
- Mäkinen MJ, George SM, Jernvall P, et al. Colorectal carcinoma associated with serrated adenoma--prevalence, histological features, and prognosis. J Pathol 2001;193:286-94. [Crossref] [PubMed]
- Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016;150:895-902.e5. [Crossref] [PubMed]
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:2088-100. [Crossref] [PubMed]
- O'Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology 2015;66:49-65. [Crossref] [PubMed]
- Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 2013;62:367-86. [Crossref] [PubMed]
- Chino A, Nagayama S, Ishikawa H, et al. Cancer emerging from the recurrence of sessile serrated adenoma/polyp resected endoscopically 5 years ago. Jpn J Clin Oncol 2016;46:89-95. [Crossref] [PubMed]
- Teriaky A, Driman DK, Chande N. Outcomes of a 5-year follow-up of patients with sessile serrated adenomas. Scand J Gastroenterol 2012;47:178-83. [Crossref] [PubMed]
- Lu FI. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol 2010;34:927-34. [Crossref] [PubMed]
- Kahi CJ. How does the serrated polyp pathway alter CRC screening and surveillance? Dig Dis Sci 2015;60:773-80. [Crossref] [PubMed]
- IJspeert JE, Vermeulen L, Meijer GA, et al. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 2015;12:401-9. [Crossref] [PubMed]


