Diminished expression of MGMT & RASSF1A genes in gastric cancer in ethnic population of Kashmir
Introduction
Gastric cancer (GC) is the fifth most common cancer in the world and is the second and third-most common cancer among males and females in Asia and worldwide (1-3), where as in Kashmir valley this cancer is 3rd in males and sixth in females (4). Carcinogenesis is a multistep process in which genetic and epigenetic alterations accumulate, a major epigenetic modification that is strongly involved in the control of gene expression and is an early landmark event in carcinogenesis is promoter region CpG island hypermethylation (5). Understanding mechanism of epigenetics holds great promise for cancer prevention, detection and therapy (6). CpG island hypermethylation at promoter site has been studied in various malignancies for several genes, and the spectrum of genes involved suggests that specific tumors may have their own distinct pattern of methylation (7). O6-methylguanine-DNA methyltransferase (MGMT) is a ubiquitous DNA repair protein that removes mutagenic and cytotoxic adducts from the O6-guanine in DNA, the preferred point of attack of many carcinogens and alkylating chemotherapeutic agents (8,9). Alkylating of DNA at the O6 position of guanine is an important step in the formation of mutations in cancer, primarily due to the tendency of the O6-methylguanine to pair with thymine during replication, resulting in a conversion of guanine-cytosine to adenine-thymine pairs in DNA (10). Furthermore, the O6 alkyl guanine-DNA adduct may cross-link with the opposite cytosine residues, blocking DNA replication (11-13) while as RASSF1A is a putative tumor suppressor gene located on 3p21 and functions in cell cycle control, microtubule stabilization, cellular adhesion, motility and apoptosis (14). Promoter region CpG island methylation, which is an epigenetic change, is the predominant mechanism of RASSF1A gene inactivation and has been recognized in many human solid tumors (15-21), thus, RASSF1A promoter methylation may be a prognostic indicator in such tumors. Several studies have shown the presence of promoter region CpG island hypermethylation and loss of protein expression (22-25), but to our best knowledge, no data concerning the expression of MGMT and RASSF1A gene and their association with promoter region CpG island hypermethylation status in GC in Kashmiri population are available. In the present study, we have studied promoter region CpG island hypermethylation of MGMT & RASSF1A and their association with expression at protein level in Kashmiri population with GC.
Methods
Study subjects
This hospital based case-control study includes 200 GC patients consisted of 117 males and 83 females from 20 to 80 years of age group. Cases and matched control tissue sample from these patients were collected from the operation theatre of Department of Surgery, Government SMHS Hospital Srinagar. Written informed consent was obtained from each participant and was carried out in accordance with the principles of the Helsinki Declaration of the world medical association. There were no age, gender, or stage restrictions, but patients with other malignant disease and not belonging to Kashmir were excluded. Only histopathologically confirmed cases and matched controls were included in study.
Molecular analysis
Genomic DNA from Cases and controls were extracted by kit based method (Quick- g DNATM MiniPrep supplied by ZYMO RESEARCH) in Molecular Biology Lab. of Department of Biochemistry, Government Medical College Srinagar (26).
Bisulphite modification and methylation-specific polymerase chain reaction (MS-PCR)
DNA methylation patterns in the CpG islands of promoter region of genes were determined by chemical treatment with sodium bisulfate and subsequent MSPCR (27). The Bisulphite modification was done by an EZ DNA Methylation–DirectTM Kit supplied by ZYMO Research (28). The primers used were listed in the literature (11,29,30). For MS-PCR, the total reaction volume was 25 µL containing 2.5 µL of 1 × Taq buffer, 1.5 µL dNTPs (1.25 mM/L), 2 µL of each forward and reverse primer (150 ng/reaction), 1.25 µL modified DNA (50 ng/reaction), 1 µL Taq DNA Polymerase (1 U/µL) and 14.75 µL De ionized water. PCR amplification was achieved using a Thermal cycler (Gradient thermal cycler from Eppendorf Master cycler pro) and reactions were hot-started at 95 °C for 10 min, followed by addition of Taq Polymerase, followed by 35 cycles of melting (95 °C for 45 s), annealing (59 °C for 45 s) and extension (72 °C for 45 s) and by final extension step at 72 °C for 4 min for MGMT gene and for RASSF1A gene the PCR conditions consisted of one incubation of 15 min at 95 °C, followed by 40 cycles of a 30 s denaturation at 94 °C, 50 s at an annealing temperature (64 °C for methylated and 59 °C for unmethylated specific primers), and a 30 s extension at 72 °C, and a final extension at 72 °C for 10 min. DNA from normal lymphocytes used as negative Control while as methylated DNA as positive Control (ZYMO RESEARCH)). 10 µL of each MS-PCR product was directly loaded on 5% agarose and visualized under UV illumination.
Western blotting
Fifty mg of GC tissue mixed with 500 µL of Trypsin-EDTA (1×), heated for 5–10 min at 37 °C, followed by centrifugation for 1 min and supernatant was discarded. Addition of 250 µL of ice-cold lysis buffer and protease inhibitors to pellet again incubated on ice for 45 min with vortexing after every 10 min. Again Centrifugation and Supernatant containing the protein was collected. Protein concentration was then measured using Nano-drop spectrophotometer and normalization was carried out with appropriate dilution to make the concentration of the proteins equal in all cases (100 µg) and was subject to SDS-polyacrylamide gel electrophoresis (12%) and transferred to polyvinylidene difluoride (PVDF) filter membranes for 30 min by blotting. The PVDF membrane was incubated for 1.5 h in blocking solution at room temperature, followed by overnight incubation at room temperature with primary antibody to MGMT and RASSF1A 1:3,000 (mouse polyclonal antibody to MGMT and RASSF1A (NP_002403.1 and NP_009113.3 Sigma Aldrich, Inc. Spruce Street, Saint Louis, USA). The excess antibody was removed, followed by 2 h incubation with anti-mouse secondary antibody (LI-COR, USA) with dilution factor 1:30,000. The PVDF membranes were washed again and the immunoreactive bands were detected by enhanced chemiluminescence and visualized by image analysis system (Bio-Rad Laboratories, Inc. Hercules, CA, USA). β-actin (Abcam Biotechnology, Cambridge, UK) was used as loading control.
Data analysis and statistics
The χ2-test with Odds ratio was used to examine the differences in the distribution of hypermethylation in promoter regions of both the genes between cases and controls and Fishers exact test was used for studying the male and female groups using Graph Pad Prism Software Version 6.0 by Graph Pad Software 2236, Avenida de la Playa, La Jolla, CA 92037, USA.
Results
Relationship between hypermethylation and GC
In GC, promoter region CpG island hypermethylation of MGMT gene was detected in 46.5% cases and 5.5% controls while as in RASSF1A gene 44% in cases and 4.5% in controls. A positive correlation between promoter region CpG island hypermethylation and GC was found (P<0.0001) suggesting that promoter region CpG island hypermethylation may play an important role in GC.
Relationship between hypermethylation and clinicopathological parameters
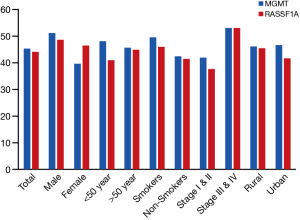
The clinicopathological data were tested for correlation with the methylation results in both genes but no significant correlation were observed between the presentation of promoter region CpG island hypermethylation in GC and patient age, gender, smoking, residence but a positive correlation was seen between CpG island hypermethylation and pathological stage in both genes (Table 1, Figures 1-3).
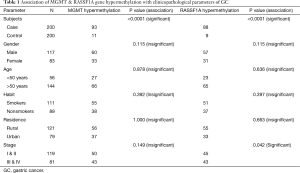
Full table
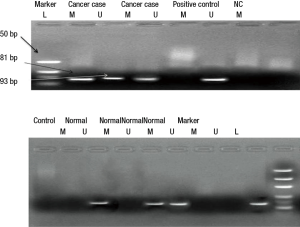
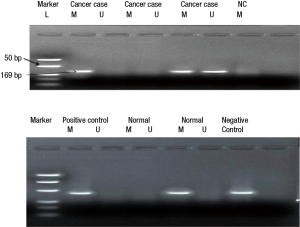
Relationship between hypermethylation and protein expression in GC
MGMT protein expression was found in 73% (79 of 107) non-hypermethylated cases and only 30% (28 of 93) in hypermethylated cases while as RASSF1A protein expression was 74% (83 of 112) in non-hypermethylated and only 36% (41 of 88) in hypermethylated cases. Statistically association of promoter region CpG island hypermethylation of MGMT & RASSF1A and down regulation of respective proteins in GC was found significant (P<0.05) (Figures 4,5).
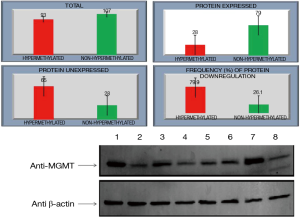
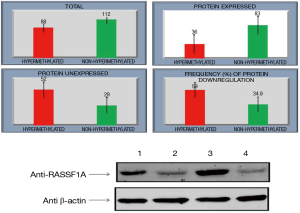
Discussion
In spite of the gradually declining incidence of GC worldwide in recent years, it still remains the fourth most common cancer and the second leading cause of cancer related deaths worldwide (31). This Cancer shows a male preponderance in almost all countries, with rates two to four times higher among males than females (32) which are in concordance with our study in which males are almost double than females. MGMT & RASSFIA gene, serving as molecular switches in pivotal processes governing cellular growth and differentiation. Several molecular alterations in these genes have been identified such as minisatellites, mutation and SNP, however epigenetic alterations have been acknowledged as an important mechanism contributing to early carcinogenesis. These genes are probably inactivated by mechanisms of methylation. Since the research on epigenetic alteration (hypermethylation) in the above mentioned genes is rare, in the present study we provided evidence that MGMT & RASSF1A expression in GC was reduced at protein level and the methylation in promoter region of the above genes was 3–5-fold more frequent in primary GC than in corresponding normal tissues. The frequency of hypermethylation of both genes showed male predominance in GC which is in concordance with other Study (33). It has been documented that the incidence of GC is known to increase with age (34) which is in concordance with our study where we found frequency of hypermethylation of both genes was high in older aged patients but Statistically association of hypermethylation with age in GC was insignificant in both genes (P>0.05). The study conducted by different authors (35,36) have shown association between the cigarette smoking and GC risk while as in our study frequency of hypermethylation was found high in smokers with GC of both genes (P>0.05). On the basis of pathological stage of GC, frequency of promoter region hypermethylation of both genes was found high in advanced stage (III & IV) compared to early stage. These results are also in concordance with the Study conducted by other authors (37,38). MGMT & RASSF1A protein was 70% and 60% down regulated in hypermethylated GC cases while as 26% each in non-hypermethylated GC cases (P<0.05). Our study showed that in the corresponding normal tissues, the methylation was seen which may be due to the difference of the samples which we studied were chronic gastritis tissues, especially intestinal metaplasia tissues in corresponding adjacent normal tissues. The study conducted by To et al. (25) reported that several genes were frequently methylated in chronic gastritis, especially in intestinal metaplasia tissues. In conclusion, Assessment of both these genes methylation status is clinically useful for identifying GC patients with a poor prognosis. In future, molecular therapies that mediate demethylation of these genes may improve patient prognosis. Less than 25% of GC cases are diagnosed at an early stage, and the 5-year survival rate is approximately 20–25% worldwide. However, the survival rate improves to over 60% if the disease is detected at an early stage, emphasizing the importance of making an early diagnosis of GC.
Acknowledgements
The authors acknowledge the support provided by Department of Biochemistry university of Kashmir and Department of Biochemistry, Government Medical College Srinagar (Research Centre University of Kashmir) Jammu & Kashmir-INDIA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was in accordance with the principles of the Helsinki Declaration of the world medical association and written informed consent was obtained from all patients.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- American Cancer Society. Detailed Guide: what is cancer, stomach cancer and its statistics? 2015. Available online: www.cancer.org
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Rasool MT, Lone MM, Wani ML, et al. Cancer in Kashmir, India: burden and pattern of disease. J Cancer Res Ther 2012;8:243-6. [Crossref] [PubMed]
- Miyamoto K, Ushijima T. Diagnostic and therapeutic applications of epigenetics. Jpn J Clin Oncol 2005;35:293-301. [Crossref] [PubMed]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002;3:415-28. [PubMed]
- Baylin SB, Esteller M, Rountree MR, et al. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 2001;10:687-92. [Crossref] [PubMed]
- Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene 2004;23:1-8. [Crossref] [PubMed]
- Esteller M. Cancer epigenetics: DNA methylation and chromatin alterations in human cancer. Adv Exp Med Biol 2003;532:39-49. [Crossref] [PubMed]
- Costello JF, Plass C. Methylation matters. J Med Genet 2001;38:285-303. [Crossref] [PubMed]
- Esteller M, Hamilton SR, Burger PC, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 1999;59:793-7. [PubMed]
- Bhakat KK, Mitra S. CpG methylation-dependent repression of the human O6-methylguanine-DNA methyltransferase gene linked to chromatin structure alteration. Carcinogenesis 2003;24:1337-45. [Crossref] [PubMed]
- Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol 2003;23:206-15. [Crossref] [PubMed]
- Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res 2005;65:3497-508. [Crossref] [PubMed]
- Wang J, Wang B, Chen X, et al. The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis 2011;32:411-6. [Crossref] [PubMed]
- Saito K, Kawakami K, Matsumoto I, et al. Long interspersed nuclear element 1 hypomethylation is a marker of poor prognosis in stage IA non-small cell lung cancer. Clin Cancer Res 2010;16:2418-26. [Crossref] [PubMed]
- Buckingham L, Penfield Faber L, Kim A, et al. PTEN, RASSF1 and DAPK site-specific hypermethylation and outcome in surgically treated stage I and II nonsmall cell lung cancer patients. Int J Cancer 2010;126:1630-9. [PubMed]
- Göbel G, Auer D, Gaugg I, et al. Prognostic significance of methylated RASSF1A and PITX2 genes in blood- and bone marrow plasma of breast cancer patients. Breast Cancer Res Treat 2011;130:109-17. [Crossref] [PubMed]
- Jiang Y, Cui L, Chen WD, et al. The prognostic role of RASSF1A promoter methylation in breast cancer: a meta-analysis of published data. PLoS One 2012;7:e36780. [Crossref] [PubMed]
- Miranda E, Destro A, Malesci A, et al. Genetic and epigenetic changes in primary metastatic and nonmetastatic colorectal cancer. Br J Cancer 2006;95:1101-7. [Crossref] [PubMed]
- Oliveira C, Velho S, Domingo E, et al. Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur preferentially in MSI sporadic colorectal cancer. Oncogene 2005;24:7630-4. [Crossref] [PubMed]
- Byun DS, Lee MG, Chae KS, et al. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res 2001;61:7034-8. [PubMed]
- Kang GH, Lee S, Kim JS, et al. Profile of aberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest 2003;83:519-26. [Crossref] [PubMed]
- Kang GH, Lee S, Kim WH, et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol 2002;160:787-94. [Crossref] [PubMed]
- To KF, Leung WK, Lee TL, et al. Promoter hypermethylation of tumor-related genes in gastric intestinal metaplasia of patients with and without gastric cancer. Int J Cancer 2002;102:623-8. [Crossref] [PubMed]
- Wani HA, Beigh MA, Amin S, et al. Methylation profile of promoter region of p16 gene in colorectal cancer patients of Kashmir valley. J Biol Regul Homeost Agents 2013;27:297-307. [PubMed]
- Herman JG, Graff JR, Myöhänen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996;93:9821-6. [Crossref] [PubMed]
- Bhat AA, Wani HA, Khan H, et al. Aberrant promoter region hypermethylation of hmlh1 Gene in Esophageal Cancer Patients of Kashmir valley. Int J Eng Sci 2012;1:24-31.
- Burbee DG, Forgacs E, Zöchbauer-Müller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst 2001;93:691-9. [Crossref] [PubMed]
- Ye M, Xia B, Guo Q, et al. Association of diminished expression of RASSF1A with promoter methylation in primary gastric cancer from patients of central China. BMC Cancer 2007;7:120. [Crossref] [PubMed]
- Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J 2014;55:621-8. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Wang YC, Yu ZH, Liu C, et al. Detection of RASSF1A promoter hypermethylation in serum from gastric and colorectal adenocarcinoma patients. World J Gastroenterol 2008;14:3074-80. [Crossref] [PubMed]
- Theuer CP, de Virgilio C, Keese G, et al. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg 1996;172:473-6. [Crossref] [PubMed]
- González CA, Pera G, Agudo A, et al. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer 2003;107:629-34. [Crossref] [PubMed]
- Smyth EC, Capanu M, Janjigian YY, et al. Tobacco use is associated with increased recurrence and death from gastric cancer. Ann Surg Oncol 2012;19:2088-94. [Crossref] [PubMed]
- Lee MG, Kim HY, Byun DS, et al. Frequent epigenetic inactivation of RASSF1A in human bladder carcinoma. Cancer Res 2001;61:6688-92. [PubMed]
- Hibi K, Sakata M, Yokomizo K, et al. Methylation of the MGMT gene is frequently detected in advanced gastric carcinoma. Anticancer Res 2009;29:5053-5. [PubMed]