Small cell carcinoma of the anus in the setting of prior squamous dysplasia and carcinoma in situ
Introduction
Squamous cell carcinoma of the anal canal is well known to develop from squamous dysplasia and carcinoma in situ (CIS). By contrast, there is no such known association with small cell carcinoma, which is rarely seen in the anal canal. We present a case of a patient with a history of anal condylomata and CIS who subsequently developed small cell carcinoma of the anal canal.
Case report
A 49-year old male with a history of human immunodeficiency virus (HIV) infection had initially presented 11 years ago with itching and burning in the perianal region. He was diagnosed at that time with anal condylomata, and since then he has undergone several surgeries for excision of anal condylomata and repair of anal fistulae. Approximately eight years after the diagnosis of anal condylomata, pathology from a condyloma excision demonstrated the presence of high grade squamous dysplasia and CIS. Two years later (approximately 10 years after the original diagnosis of anal condylomata), the patient developed a right anterior lateral fistula of the anus with recurrent condylomata, and excision was performed with pathology again demonstrating high grade squamous dysplasia. Within one month of his surgery, the patient developed severe rectal pain and bleeding. Physical exam was limited at this time due to patient discomfort. Magnetic resonance imaging of the pelvis demonstrated circumferential thickening of the anal canal measuring up to 1.7-cm, along with an 8.0-cm × 6.3-cm heterogeneously enhancing left perirectal mass consistent with a lymph node conglomerate. Examination under anesthesia and proctoscopy were performed, demonstrating a 3.0-cm nodule at the anal verge associated with an 8.0-cm area of ulceration that extended to the distal rectum. Biopsies of the nodule and throughout the area of ulceration were performed. Pathology demonstrated the presence of a high grade neuroendocrine carcinoma, small cell type, with associated squamous CIS (Figures 1,2). Immunostains were diffusely positive for synaptophysin and p16, focally positive for high molecular weight keratin, and negative for chromogranin and p63. Testing for high-risk human papillomavirus (HPV) was not performed. Of note, the patient’s absolute CD4 lymphocyte count one month prior to biopsy was 754 cells/µL. Positron emission tomography-computed tomography (PET/CT) was performed for staging, revealing hypermetabolic activity in the primary anal/rectal mass along with apparent metastatic lesions in the perirectal and inguinal lymph node regions (Figure 3). Additional hypermetabolic activity was noted in the paraaortic and prevascular lymph node regions, but these findings were thought to be inflammatory in etiology rather than metastatic. The patient received two cycles of induction chemotherapy with cisplatin 80 mg/m2 and etoposide 100 mg/m2 on days one to three separated by 21 days between cycles. He then received a course of radiation therapy to the primary anal canal tumor and the inguinal, perirectal, and pelvic lymph nodes to a dose of 54.0 Gy with two further cycles of concurrent cisplatin 80 mg/m2 and etoposide 100 mg/m2 on days one to three separated by 28 days between cycles. During his treatment, the patient developed grade three neutropenia, grade three dermatitis, and grade two diarrhea, but he was able to complete his treatments as planned. At the completion of treatment, the patient developed perineal and inguinal abscesses consistent with hidradenitis suppurativa. These were treated with incision and drainage, and biopsy of these lesions demonstrated the presence of suppurative inflammation within the setting of granulation tissue and squamous epithelium but no evidence of malignancy. PET/CT performed approximately three months following the completion of the patient’s chemoradiation demonstrated marked interval improvement in the size and metabolic activity of the disease in the patient’s anal canal and regional lymph node regions (Figure 4), and the finding of hypermetabolic activity in the mediastinum had resolved completely. At the time of his last follow-up visit (five months after completion of chemoradiation), the patient’s pain and swelling in the anal canal had improved significantly. Additionally, a follow-up sigmoidoscopy demonstrated no evidence of residual or recurrent tumor, and biopsies of an area of mucosal irritation within the rectum were negative for malignancy.
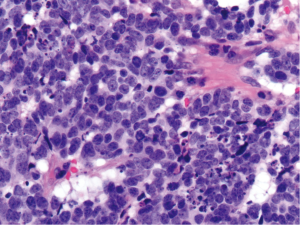
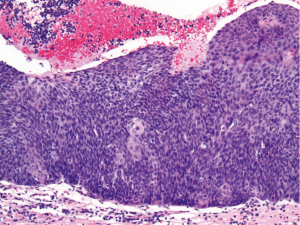
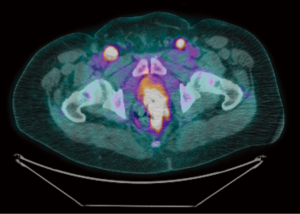
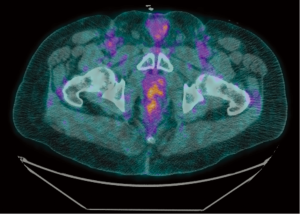
Discussion
Approximately 5,820 cases of squamous cell carcinoma of the anus were diagnosed in the United States in 2011 (1). There is a strong association between squamous cell carcinoma of the anus and high-risk subtypes of HPV, most notably HPV-16 and HPV-18 (2). Infection with HIV is a prominent risk factor for the development of squamous cell carcinoma of the anus, and the prevalence of anal cancer in patients with HIV infection is significantly higher than that of the general population (3).
In contrast to squamous cell carcinoma, small cell carcinoma of the anal canal is extremely rare. A recent study that collected data from population-based registries covering approximately 83% of the U.S. population identified only 210 cases of small cell carcinoma of the anus between 1998 and 2003 (4). While small cell carcinomas of the head and neck and the cervix have known associations with HPV (5,6), the relationship between HPV and small cell carcinoma of the anus is largely undefined to date. There is data, however, to suggest that a relationship between these entities may exist. A recent study by Cimino-Mathews et al. aimed to determine the prevalence of high-risk HPV (including HPV-16 and HPV-18) in the tumors of 16 patients with small cell carcinoma of the anus (n=5) or rectum (n=11) at Johns Hopkins University. The investigators found that 100% of tumors in both the anus and rectum were strongly positive for high-risk HPV by immunohistochemistry (using nuclear and cytoplasmic p16 as a surrogate marker) and that 100% of the anal tumors and 82% of the rectal tumors were positive for high-risk HPV by in situ hybridization (7). These results suggest that HPV infection is a component of the pathogenesis of small cell carcinoma of the anus.
In addition to the cases identified in the Johns Hopkins study, five individual case reports of small cell carcinoma of the anus have been published within the English language literature to date (Table 1) (8-12). Notably, ours is the first report of a patient with small cell carcinoma of the anus in the setting of previously identified squamous dysplasia and/or CIS, although there is one additional case report of a patient with neuroendocrine carcinoma of the anus with associated squamous intraepithelial neoplasia and molecular studies positive for HPV-18 (13). Our patient’s clinical course, in conjunction with the data from Cimino-Mathews et al., suggest that, in a similar manner to that of squamous cell carcinoma, small cell carcinoma of the anus may develop linearly from squamous dysplasia and CIS.
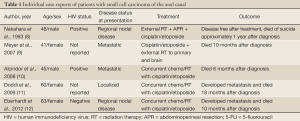
Full table
While the relationship between HIV and squamous cell carcinoma of the anus is well established, the role of HIV infection in the pathogenesis of small cell carcinoma of the anus is unclear. A recent review of the literature identified only two reported cases of small cell carcinoma of the anus in a patient with known HIV infection (8,10). It is conceivable that chronic immunosuppression associated with HIV infection may contribute to a tumor microenvironment that facilitates tumor progression, growth, and dedifferentiation. While these relationships are far from established, it may be the case that small cell carcinoma of the anus is more likely to develop in such a microenvironment and that HIV infection is indeed a risk factor for this malignancy.
Since small cell carcinoma of the anus is an extremely rare malignancy, a definitive understanding of its pathogenesis is not established. As more data accumulates to suggest a relationship between HPV infection and the development of small cell carcinoma of the anus, HPV-directed therapies could prove beneficial. Furthermore, vaccines against high-risk HPV may be protective against the development of this aggressive cancer. Finally, the role of HIV in the pathogenesis of small cell carcinoma of the anus is unclear and merits further study. Ultimately, more research is needed to more clearly delineate the relationships between HPV, HIV, and small cell carcinoma of the anus.
Acknowledgements
Disclosure: There are no financial disclosures for any authors on this study. This study had no financial support.
References
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36.
- Hoots BE, Palefsky JM, Pimenta JM, et al. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009;124:2375-83.
- Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008;148:728-36.
- Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer 2008;113:2892-900.
- Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol 2011;35:1679-84.
- Wang HL, Lu DW. Detection of human papillomavirus DNA and expression of p16, Rb, and p53 proteins in small cell carcinomas of the uterine cervix. Am J Surg Pathol 2004;28:901-8.
- Cimino-Mathews A, Sharma R, Illei PB. Detection of human papillomavirus in small cell carcinomas of the anus and rectum. Am J Surg Pathol 2012;36:1087-92.
- Nakahara H, Moriya Y, Shinkai T, et al. Small cell carcinoma of the anus in a human HIV carrier: report of a case. Surg Today 1993;23:85-8.
- Meyer A, Bruns F, Richter K, et al. Small cell cancer of the anal canal--case report of a rare tumor. Anticancer Res 2007;27:1047-50.
- Alcindor T, Tosikyan A, Vuong T, et al. Small-cell anal carcinoma and AIDS: case report and review of the literature. Int J Colorectal Dis 2008;23:135-6.
- Doddi S, Singhal T, De Silva C, et al. Small cell carcinoma of the anus: a case report. Cases J 2009;2:9396.
- Eberhardt JM, Brown K, Lo S, et al. Extrapulmonary small cell carcinoma of the anal canal: a case report and review of the literature. Case Report Med 2012;2012:341432.
- Ohtomo R, Sekine S, Taniguchi H, et al. Anal canal neuroendocrine carcinoma associated with squamous intraepithelial neoplasia: a human papillomavirus 18-related lesion. Pathol Int 2012;62:356-9.


