Is it time to split strategies to treat homologous recombinant deficiency in pancreas cancer?
Introduction
Pancreatic adenocarcinoma is the 12th most commonly diagnosed cancer in the United States but it disproportionately represents the 5th most common cause of cancer-related death (1). Unlike many other cancer types, a rise in incidence and mortality rates from pancreatic adenocarcinoma have been noted (2). In fact, it was projected that by 2030, pancreatic cancers will surpass breast, colorectal and prostate cancers to become one of the leading causes of cancer-related deaths, and therefore incurring a significant public health burden (3). A majority of cases present as either locally advanced or metastatic disease, where the disease is invariably fatal (4). Key oncogenic pathways such as RAS-MAPK, PI3KCA and TGF-beta signaling pathways have been identified in pancreatic cancers (5) and targeted in numerous drug trials although efforts to date have been unfruitful (6).
Germline mutations in BRCA1 (Breast Cancer 1, Early Onset) and BRCA2 (Breast Cancer 2, Early Onset) genes significantly increase the carrier’s lifetime risk for breast and ovarian cancers, and are defining features of hereditary breast and ovarian cancer syndromes (7). BRCA1/2-related ovarian and breast cancer represent a clinically significant subset, as characterized by specific phenotypic manifestations, such as triple negative or basal-like breast cancers (8,9) and an increased risk for visceral involvement in ovarian cancers (10-12). More importantly, these correlations carry therapeutic relevance, as BRCA1/2-related breast and ovarian cancers demonstrate increased susceptibility to platinum-based chemotherapy and poly (ADP-ribose) polymerase (PARP) inhibition (13-18). However, larger-scale studies soon recognized that BRCA1 and BRCA2 increase the risk of other cancer types as well. The link between prostate cancer and BRCA1/2 mutations has been established (19,20) and worse clinical outcomes have been suggested (21-23). Not unlike observations in ovarian and breast cancers, a recent phase II study has shown that these prostate cancers demonstrated heightened response to PARP inhibition (23).
A similar and parallel picture with pancreatic adenocarcinoma is emerging (19,20), BRCA-related pancreatic adenocarcinoma might exhibit unique biology and natural history. In fact, it might represent components of a subset of pancreatic adenocarcinoma defined by underlying molecular biology in defective homologous recombination mechanism, as seen in other disease types. The available data and evidence will be reviewed.
BRCA1 and BRCA2—role in DNA damage response and repair
The genome is subject to regular and frequent stressors, from both endogenous and environmental agents (24). Constant maintenance of genomic integrity is coordinated by multiple DNA damage response and repair mechanisms (24). Damage to DNA, especially in the form of double strand breakage may be lethal for cellular survival. This form of damage can be induced by cross-linking agents or ionizing radiation, amongst other causative factors. Various cytotoxic chemotherapies exert antineoplastic effect by targeting the DNA macromolecule. Platinum compounds such as cisplatin, carboplatin and oxaliplatin bind covalently to DNA to form DNA-adducts which impede cellular processes and ultimately lead to apoptosis (25). Other non-platinum alkylators function by similar mechanisms, such as mitomycin-C and temozolomide (26). On the other hand, agents such as irinotecan and other camptothecins inhibit DNA topoisomerase I which stabilizes the Topo-1-DNA cleavage complex and blocks the religation of DNA, leading to accumulation of single strand DNA breaks and damage (27).
Double strand breaks are repaired by either the homologous recombination or non-homologous end joining pathways. Homologous recombination is the repair mechanism of choice due to lower error rates (24). Cells with defective double strand repair shows a high degree of chromosomal instability, including chromosome breaks and radial chromosomes, which may lead to acquired mutations with consequential oncogenesis (28-30). The entire mechanism is tightly regulated, beginning with DNA damage sensing and response by checkpoint kinases ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and rad3-related protein (ATR), which can lead to downstream activation of Fanconi Anemia complex. The complex is responsible for recruitment of other component proteins of the homologous recombination mechanism. BRCA1 and BRCA2 genes encode for proteins which play crucial role in homologous recombination pathway (31,32). Clinically significant mutations in these genes are frequently frameshift insertions or deletions which are translated into functionally impaired proteins (33) and thereby contributing to defective homologous recombination mechanism. The impaired mechanism leads to accumulation of mutations and chromosomal defects which increase the risk of carcinogenesis, and conversely, increased sensitivity to cross-linking properties of cytotoxic agents (34-36).
In the absence of effective double strand breakage repair, alternative DNA repair mechanisms are often recruited. PAR-1 is a nuclear protein which localizes to the site of DNA damage and contributes to the majority of PARP activity. PARP is a critical component of base excision repair pathway, an important pathway in repair of single strand breakage. Loss of PARP-1 increases the formation of DNA lesions that might be repaired by components of homologous recombination. In cells with loss of function of BRCA1/2, PARP inhibition further interrupts alternative DNA repair pathways, leading to accumulation of large numbers of chromatid aberrations and subsequent cell cycle arrest and cell death. This concept is commonly known as synthetic lethality (35). Clinically, the effect of synthetic lethality is more frequently observed in BRCA1/2-mutated cancers treated with PARP inhibitors (18,37,38).
Ultimately, the net result of dysfunctional DNA response and repair mechanisms is the accumulation of potentially deleterious mutation and consequential genomic instability (39). Besides tumorigenesis, genomic instability could also contribute to heterogeneity with diverse genetic alterations in different metastatic lesions, leading to heterogeneous response to therapy (40).
BRCA1/2 mutations in pancreatic adenocarcinoma—tip of the iceberg
The vast majority of pancreatic adenocarcinomas are sporadic in nature but emerging data from the last two decades have indicated that 5–15% of pancreatic adenocarcinoma have an inheritable component and are linked to inherited cancer susceptibility syndromes (20). Associated syndromes included hereditary breast and ovarian cancer syndrome (20), Lynch syndrome, Peutz-Jahgers, familial atypical multiple mole melanoma syndrome and hereditary pancreatitis (41).
Hereditary breast and ovarian cancer syndrome with variants in its corresponding genes BRCA1 and BRCA2 are the most studied and important syndrome related to inherited pancreatic cancer. Some of these observations stem from large population-based studies on hereditary breast and ovarian cancer syndromes. The massive Hereditary Breast Cancer Study enrolled over 5,000 female carriers of BRCA1/2 mutations, and over an average follow-up of 2 years, eight cases of pancreatic cancers were diagnosed. Albeit numerically low, the incidence was significantly higher than population average with a standardized incidence radio of 2.55 for BRCA1 carriers and 2.13 for BRCA2 carriers (42). The Breast Cancer Linkage Consortium examined 3,728 women with germline BRCA2 mutation and observed 3.51 times increased risk for development of pancreatic cancer (43).
Studies of pancreatic cancer patients with related family histories observed an enrichment of related genetic variants as well. In one of the earliest studies, 29 patients with pancreatic cancer and highly significant family history from the National Familial Pancreatic Tumor Registry (NFPTR) were subjected to germline DNA analysis for four putative tumor suppressor candidate genes. The cohort was significantly enriched with germline BRCA2 mutation in 17.2% of the patients while no germline mutations in the other three genes were observed (MP2K4, MADH4 and ACVR1B) (44). A similar study by Lucas and colleagues identified germline BRCA1/2 mutations in 21.9% of 32 patients with pancreatic cancer and 18.9% in patients without a cancer diagnosis currently enrolled in their high-risk pancreatic cancer prevention and genetics program (45). However, the majority of these early studies were significantly restricted by small sample size. In one of the largest published series to date, 175 patients with pancreatic cancer treated in Memorial Sloan Kettering Cancer Center (MSKCC) underwent clinical genetic counseling and germline DNA analysis based on suspicious personal or family history. Amongst the study cohort, 56.0% has Ashkenazi Jewish ancestry, 26.3% had personal history of prior malignancies and 30–50% had family history of malignancies in first degree relatives. In this study, a pathogenic mutation was identified in 15.1% of patients, which included 13 patients with BRCA2 mutation, four patients with BRCA1 mutations, p16 mutations in two further patients, PALB2 in one patient and four patients harbored germline mutations in mismatch repair genes (46). Rate of mutation detection for BRCA1/2 was significantly higher for patients with Ashkenazi Jewish ancestry (13.7%) compared to those with non-Ashkenazi Jewish ancestry (7.1%).
It is worth noting that these studies enrolled patients selected for strong personal or family history for pancreatic cancers or BRCA-related malignancies. In an unselected cohort of patients with pancreatic adenocarcinoma, only 11 of 306 patients (3.6%) were found to harbor pathogenic BRCA2 mutations and another 3 patients (1%) with BRCA1 mutations (47). A high rate of Ashkenazi ancestry was noted in the study cohort at 10.8%. When analyzed separately, BRCA1/2 mutations were observed in four of 13 (12.1%) Ashkenazi Jewish patients and in 10 of 273 (3.7%) non-Ashkenazi Jewish patients (P=0.05), which reflects the observation from the MSKCC series described previously.
Germline mutations in other members of homologous recombination mechanism
As seen in breast or ovarian cancers, inherited mutations in BRCA1/2 and other known genetic syndromes have not been able to entirely account for a significant majority of familial cancers. This would appear to be the case for pancreatic cancers as well. Albeit still a small body of literature, recent investigations have begun to implicate germline mutations in other components of homologous recombination as risk factors for pancreatic carcinogenesis. For example, in an unselected cohort of 96 pancreatic cancer patients from the Mayo Clinic Pancreatic Cancer patient registry, 14 pathogenic mutations in 13 patients were identified in 8 genes, namely ATM, BRCA2, checkpoint kinase 2 (CHEK2), MutS Homolog 6 (MSH6), BRCA1-Associated RING Domain 1 (BARD1), BRCA1, Fanconi Anemia, Complementation Group M (FANCM) and Nibrin (NBN), majority of which are component of the homologous recombination pathway (48). When 638 patients with familial pancreatic cancer without known germline mutations were subjected to whole genome sequencing, truncating mutations in a wide range of DNA damage repair genes were identified at low frequencies, including ATM, Polymerase (DNA Directed) Nu (POLN), Polymerase (DNA Directed) Theta (POLQ), Fanconi Anemia, Complementation Group C (FANCC), FANCM, et cetera (49).
Other groups adopted a more targeted approach, evaluating the prevalence of selected mutations. The Ontario Pancreas Cancer Study was designed to investigate the prevalence of germline mutations in a panel of genes—including ATM, BRCA1, BRCA2, APC, CDKN2A and various mismatch repair genes. Amongst 290 probands, 11 pathogenic mutations were identified, of which 3 were alterations in the ATM gene (50). In another study of the Mayo Clinic Pancreatic Cancer Cohort, four pathogenic mutations in ATM were noted out of 14 pathogenic mutations in 13/96 patients(48), while another regional pancreatic database observed four mutations in ATM out of 11 mutations seen in 10/70 patients (51), therefore conferring a prevalence of 1.0–5.7% amongst high risk patients with pancreatic cancers.
Germline PALB2 mutation has also been reported in various series. Zhen and colleagues observed deleterious mutation in PALB2 gene in 0.6% of patients with familial pancreatic cancer (52). In a large series which included 132 non-BRCA1/2 breast and ovarian cancer families with at least one confirmed case of pancreatic cancer, prevalence of germline PALB2 mutation was estimated to be approximately 1.5% (53). However, in a Dutch series of 28 non-BRCA1/2 familial pancreatic cancer families and 28 non-BRCA1/2 familial breast cancer families with at least one confirmed case of pancreatic cancer, no PALB2 mutations were detected at all (54). In a large Italian series of non-BRCA1/2 familial breast cancer families, frequency of germline PALB2 mutation was estimated to be 2.1%. However, in 39 cases with confirmed family history of pancreatic cancer, three cases of PALB2 mutation was detected, suggesting that families with both familial breast and pancreatic cancers might be enriched with PALB2 mutations in the absence of deleterious or variant germline BRCA1/2 mutations (55).
van der Heijden and colleagues observed mutations in FANCC and FANCG genes in a small subset of patients with early onset pancreatic adenocarcinoma (56). In a large screening study of 421 patients with pancreatic cancers, two truncating mutations in FANCC were observed but none were observed for FANCG although they were not associated with family history (57). Rogers and colleagues examined genomic DNA from 38 patients with familial pancreatic cancer for mutations in FANCC and FANCG genes. Several polymorphisms of indeterminate functional significance were reported. The authors observed that these genes did not appear to contribute to the clustering of pancreatic cancers seen in the setting in familial pancreatic cancer, and concluded that these genes were uncommon causes of inherited pancreatic cancers (58).
These efforts collectively indicate that familial pancreatic adenocarcinoma patients harbor germline mutations in other components of DNA damage response or homologous recombination mechanism. However, prevalence of individual mutations is low, comparable to observations in other cancer types (59-61). The low prevalence of these mutations and the relative rarity of pancreatic adenocarcinoma also render it challenging to delineate the penetrance strength of these mutations or their absolute risk in pancreatic carcinogenesis (with the exception of Roger and colleagues’ effort on FANCC and FANCG).
Beyond the germline—somatic mutations in BRCA1/2 and other homologous recombination genes
Somatic mutations in BRCA1/2 appear to be rare events in sporadic breast cancers (62,63), which is more frequently characterized by epigenetic silencing of BRCA1/2 (64). Conversely, somatic mutations in BRCA1/2 account for at least one third of overall BRCA1/2 mutations in ovarian cancer (65,66). An integrative genomic analysis in metastatic castrate resistant prostate cancer observed BRCA2 alterations in 12.5% of cases although pathogenic germline BRCA2 mutations were only observed in 5.3% of cases (67). This has not been extensively investigated in pancreatic cancer until recently due to advancement in sequencing technology and bioinformatics capability to work with very limited and small amount of genetic material typical of pancreatic cancer biopsies.
One of the earliest reports was the IMPaCT trial by the Australasian Gastrointestinal Trials Group (AGITG) which was designed as a phase II trial of gemcitabine versus tailored therapies based on actionable mutations from a limited panel of genes which included BRCA1, BRCA2, PALB2 and ATM. Of the 76 patients who completed molecular evaluation, two mutations in BRCA2 and one in ATM were detected, translating into a rate of 3.9% (68). Whole exome sequencing was performed on 99 cases of tumor tissue from early stage (stages I and II) sporadic pancreatic adenocarcinoma and five mutations in ATM were detected (5.1%) but none in BRCA1/2 or other Fanconi Anemia related genes (69).
Whole genome sequencing was performed via the Australian Pancreatic Cancer Genome Initiative and four subtypes of pancreatic cancers were recognized. In the unstable subtype which constitutes 14% of all samples, a large number of structural variation events were noted and therefore suggestive of genomic instability. Of the 14 patients in this subgroup, germline mutations in either BRCA2 or PALB2 were detected in 6 patients while somatic mutations in BRCA1/2 were noted in 5 other patients. Mutations in other DNA repair genes including RPA1, REV3l, ATM, FANCM, XRCC4 and XRCC6 were also noted across the entire cohort (70).
In a separate study of 109 micro-dissected pancreatic cancers, alterations in Fanconi Anemia genes or ATM, CHEK2, BCLAF1, BRCA1 and BRCA2 were noted in over 35% of cases (71).
Overall, the rate of somatic mutations in BRCA1/2 and related genes could range from 3.9–35% across different studies performed on different sequencing platforms. No studies to date have examined the rate of epigenetic silencing of BRCA1/2 or related genes in pancreatic adenocarcinoma. The as-yet unpublished Cancer Genome Atlas dataset includes methylation analysis and may provide a more comprehensive analysis.
Mutations in BRCA1/2 and other homologous recombination component genes as prognostic marker
Germline mutations in both BRCA1 and BRCA2 appear to confer differential survival advantage across different disease types. Large meta-analyses have suggested that BRCA1/2 carriers with ovarian cancers have better long-term outcomes compared to non-carriers (72,73) However, breast cancer patients with BRCA1 mutation appear to have shorter disease-related long-term survival while BRCA2 appeared to confer no influence on survival (72,74) Similar data for pancreatic adenocarcinoma remain limited due to relatively lower incidence of pancreatic cancer and lower frequency of related mutations.
In Witkiewicz and colleagues’ analysis of survival based on altered pathway alterations, defective homologous recombination was associated with a trend towards worse prognosis (71). In a series of 71 patients with germline BRCA1/2 mutations, 15 patients with stage I/II disease had a overall survival rate of 52% at 5-year, which is superior to historical control although the small sample size precluded the ability for any meaningful control (75). A follow-up multicenter case-controlled study by the same authors, however, did not identify improved survival. Median overall survival for twenty patients with BRCA-mutated pancreatic cancer and 40 matched wild type control were 23.8 and 25.9 months, respectively, with no statistically significant differences (76).
Non-BRCA1/2 components of homologous recombination mechanism might exert prognostic influence as well. A large multi-institutional evaluation of 396 surgically resected pancreatic adenocarcinomas identified biallelic loss of ATM in 12.8% of cases, which was associated with worse overall survival (77). In a study of 119 patients, single nucleotide polymorphisms in ATM, ATR, CHEK1 and CHEK2 demonstrated significant combined genotypic effect on survival, with more polymorphisms present per patient correlating with shorter overall survival (78). Various separate analyses from investigators in MD Anderson Cancer Center also identified similar observations—presence of single nucleotide polymorphisms in POLB, OGG1, APEX1 and XRCC1 were cumulatively adverse to overall survival (79-81).
Chemotherapy for advanced pancreatic adenocarcinoma
Systemic therapy for advanced pancreatic adenocarcinoma has predominantly been gemcitabine-based regimens, since the demonstration of its superiority over 5-fluorouracil (82). Attempts at pairing gemcitabine to various agents, including cisplatin or oxaliplatin, have not been able to improve overall survival in large phase III trials conducted in unselected patients. The French PRODIGE intergroup in 2011 was able to demonstrate significant survival and clinical benefit from FOLFIRINOX in comparison to gemcitabine. With a total of 342 patients randomized, the study was able to show significant superior median progression-free and overall survival in the FOLFIRINOX arm (6.4 and 11.1 months) compared to patients who were treated with gemcitabine (3.3 and 6.8 months) (83). The Metastatic Pancreatic AdenoCarcinoma Trial (MPACT) study randomized 861 patients with metastatic disease and a Karnofsky performance score (KPS) of 70–100% to single-agent gemcitabine or gemcitabine plus nab-paclitaxel (84). Median progression-free survival was superior in the combination arm, at 5.5 vs. 3.7 months. Overall survival was also significantly improved from 6.7 to 8.5 months. These two trials have redefined the therapeutic landscape of advanced pancreatic adenocarcinoma and became the new standards of care. Response rates with these new regimens were 31.6% and 23.0%, respectively, clearly indicative of differential extent of responses between individuals. However, predictive clinical factors or biomarkers remain sorely lacking.
Can homologous recombination deficiency (HRD) is used as a predictive marker of chemotherapy response?
In a panel of patient-derived pancreatic cancer xenografts, BRCA-mutant xenografts were significantly more sensitive to cisplatin than BRCA-wild type xenografts (85). Clinically, considerable amount of evidence have been demonstrated in ovarian cancer (15,86,87) and breast cancer (13,14,88).
For pancreatic cancer, early indications that patients harboring BRCA1/2 mutations might respond differentially to platinum agents were published as individual case reports (89-92). Sonnenblick and colleagues reported the case of a 60-year-old carrier of a rare BRCA2 germline mutation. The patient was treated initially with gemcitabine with progressive disease as best response. Cisplatin was added to gemcitabine, which led to a dramatic complete response radiographic and biochemically (89). Chalasani and colleagues reported the case of a 49-year-old woman with germline BRCA2 mutation and metastatic pancreatic adenocarcinoma who achieved substantial partial response with third line chemotherapy with capecitabine and mitomycin C, a cross-linking agent with identical mode of action as cisplatin (90). Another case reported a 71-year-old BRCA2 carrier with dual diagnosis of prostate and pancreatic adenocarcinomas, who achieved disease control with second-line irinotecan for over 27 cycles of treatment (91).
In a small series of ten patients with pancreatic adenocarcinoma and germline BRCA2 mutation (93), six patients received platinum-based combination chemotherapy and achieved a mean duration of response of 4.8 months, ranging from 2 to 8 months. For the seven patients who received a topoisomerase-I inhibitors alone or in combination with other agents, mean duration of response of 8.3 months were observed, while the two patients who were exposed to mitomycin-C responded for 2.3 and 3 months, respectively. Although the sample size was small and patients were highly selective, these data suggested that superior disease control might be attainable in patients with BRCA1/2 mutations. In another small series of 15 patients, 6 patients received platinum-based chemotherapy as first-line therapy, five of those experienced partial radiographic response, including one complete response with FOLFIRINOX (19). Golan and colleagues reported superior overall survival in platinum-treated patients with either stage III disease (48 vs. 10 months) or stage IV disease (15 vs. 7 months) (75).
Homologous recombination defect as a molecular target with PARP inhibitors
The concept of synthetic lethality (35) has led to effective use of PARP inhibitors in other BRCA-related cancers (18,37,38). In pancreatic cancer, pre-clinical studies have reaffirmed comparable observations. In pancreatic cancer xenografts, increased sensitivity to cisplatin but not gemcitabine was observed in BRCA1/2-mutated tumors (85). In murine models, the addition of PARP inhibitor to cisplatin could increase time to cancer and overall survival (94).
Early clinical data have been promising. In a small retrospective series of 15 patients with pancreatic cancer and germline BRCA mutations (4 with BRCA1 and 11 with BRCA2), two patients were treated with PARP inhibitors either alone or in combination with chemotherapy in the first line non-curative setting, and enjoyed partial response lasting 2 and 6 months, respectively. Of two patients who were treated with PARP inhibitors in the second line setting, one sustained stable disease for 6 months (19).
At present, several PARP inhibitors are in various stages of investigation in pancreatic adenocarcinoma, namely olaparib, veliparib and rucaparib.
In a large phase II study, patients with advanced solid tumor cancers and confirmed germline BRCA1/2 mutations were treated with single agent olaparib at 200 mg BID. For the 23 patients with gemcitabine-refractory metastatic pancreatic cancer, a response rate of 21.7% was observed while stable diseases of at least 8 weeks were observed in 34.8%. Median progression-free and overall survivals were 4.6 and 9.8 months, respectively (95). In a phase I study of gemcitabine and olaparib, the recommended phase 2 dose was determined to be gemcitabine 600 m/mg2 and olaparib 100 mg BID. The study included a dose expansion cohort where patients with treatment-naïve locally-advanced or metastatic pancreatic cancer were randomized to olaparib plus gemcitabine at the maximum tolerated dose as determined or standard dose gemcitabine. The combination did not appear to confer improvement in disease control or survival rates, however, patients were not routinely genotyped in this study (96). Although no further development was planned for the combination, olaparib is currently being investigated various other clinical settings for pancreatic cancer, including: in a phase 1 study of olaparib in combination with irinotecan, cisplatin and mitomycin-C (NCT01296763), and in the phase III POLO trial (NCT02184195). The trial aims to accrue approximately 145 patients with confirmed deleterious germline BRCA1/2 mutations who achieve at least disease stabilization following 16 weeks or more of platinum-based chemotherapy. Patients will be randomized in a 3:2 ratio to olaparib as maintenance therapy or placebo. The primary endpoint is progression-free survival (Table 1).
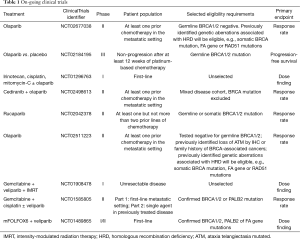
Full table
Some clinical activity for single agent veliparib was also observed. In an early phase study, a total of 16 patients with confirmed BRCA1/2 or PALB2 mutation and pre-treated pancreatic cancer were enrolled. Five had BRCA1 mutation while 11 had BRCA2 mutations. Only one partial response was noted which progressed at 6 months, while four patients achieved stable disease and ten had progressive disease as best response (97). Veliparib was also examined in combination with cisplatin and gemcitabine in a phase IB dose finding study (98). A total of 17 patients were enrolled, and the randomized phase II dose of veliparib was determined to be 80 mg PO BID days 1–12 combined with cisplatin 25 mg/m2 and gemcitabine 600 mg/m2 IV days 3 and 10, every 21 days. Nine patients had BRCA1/2 mutation while the remaining patients were BRCA wild type and enrolled based on strong personal or family history of malignancy. No significant activity was noted in the latter group but for the BRCA-mutated subgroup, 6 out of 9 patients experienced partial response while the remaining patients had stable disease as best response. A randomized phase II of cisplatin, gemcitabine plus veliparib versus cisplatin and gemcitabine is currently recruiting (NCT01585805). Other on-going veliparib trials in pancreatic cancers include a phase I study of veliparib in combination with gemcitabine and intensity modulated radiation therapy in patients with locally-advanced disease (NCT01908478) and a phase I/II study of veliparib in combination with FOLFOX in metastatic pancreatic adenocarcinoma (NCT01489865).
Rucaparib is another PARP inhibitor in a much earlier stage of development. A phase II trial is currently on-going where patients with metastatic pancreatic adenocarcinoma with known somatic or germline BRCA1/2 mutation and at least one prior line of systemic therapy were treated with Rucaparib (NCT02042378).
Conclusions
There is now ample evidence confirming that deleterious mutations in BRCA1/2 and other homologous recombination component genes occur in a sizeable minority of pancreatic adenocarcinomas. Tumors secondary to germline BRCA1/2 mutations appear to mirror those of breast, ovarian and prostate cancer in terms of gene penetrance, risk of tumorigenesis, and in particular, susceptibility to DNA-targeting cytotoxic agents. PARP inhibition, similarly, has generated early promising signals.
However, many questions still remain. As has been discussed above, the reported prevalence of germline mutations in other homologous recombination component genes is low and the deleterious effect of individual genes in carcinogenesis, prognosis and therapeutic implication has not been validated although observations in other disease types might suggest comparable efficacy to therapies (38).
Therefore, a subtype of pancreatic adenocarcinoma as characterized by defective homologous recombination exists, and it is emerging as a subset for which defined treatment options may exist. There is ample venue for further investigation either in the form of prospective studies or correlative evaluation from existing trials. We are cautiously optimistic that HR deficiency represents a marker of potential actionability for pancreatic adenocarcinoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- O'Reilly EM. Evolving Panorama of Treatment for Metastatic Pancreas Adenocarcinoma. J Clin Oncol 2013;31:1621-3. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Knudsen ES, O'Reilly EM, Brody JR, et al. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology 2016;150:48-63. [Crossref] [PubMed]
- Sahin IH, Iacobuzio-Donahue CA, O'Reilly EM. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert Opin Ther Targets 2016;20:341-59. [Crossref] [PubMed]
- King MC, Marks JH, Mandell JB, et al. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003;302:643-6. [Crossref] [PubMed]
- Foulkes WD, Brunet JS, Stefansson IM, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res 2004;64:830-5. [Crossref] [PubMed]
- Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 2003;95:1482-5. [Crossref] [PubMed]
- Muggia F, Safra T. 'BRCAness' and its implications for platinum action in gynecologic cancer. Anticancer Res 2014;34:551-6. [PubMed]
- Lorusso D, Scambia G, Pignata S, et al. Prospective phase II trial of trabectedin in BRCA-mutated and/or BRCAness phenotype recurrent ovarian cancer patients: the MITO 15 trial. Ann Oncol 2016;27:487-93. [Crossref] [PubMed]
- Gourley C, Michie CO, Roxburgh P, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol 2010;28:2505-11. [Crossref] [PubMed]
- Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2015;33:1902-9. [Crossref] [PubMed]
- Telli ML, Jensen KC, Vinayak S, et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib As Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. J Clin Oncol 2015;33:1895-901. [Crossref] [PubMed]
- Gorodnova TV, Sokolenko AP, Ivantsov AO, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett 2015;369:363-7. [Crossref] [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376:235-44. [Crossref] [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [Crossref] [PubMed]
- Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015;16:87-97. [Crossref] [PubMed]
- Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 2011;16:1397-402. [Crossref] [PubMed]
- Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121:269-75. [Crossref] [PubMed]
- Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol 2015;68:186-93. [Crossref] [PubMed]
- Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748-57. [Crossref] [PubMed]
- Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res 2010;16:2115-21. [Crossref] [PubMed]
- Houtgraaf JH, Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med 2006;7:165-72. [Crossref] [PubMed]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573-84. [Crossref] [PubMed]
- Drabløs F, Feyzi E, Aas PA, et al. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389-407. [Crossref] [PubMed]
- Sakasai R, Iwabuchi K. The distinctive cellular responses to DNA strand breaks caused by a DNA topoisomerase I poison in conjunction with DNA replication and RNA transcription. Genes Genet Syst 2016;90:187-94. [Crossref] [PubMed]
- Zhu C, Mills KD, Ferguson DO, et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell 2002;109:811-21. [Crossref] [PubMed]
- Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 2012;47:497-510. [Crossref] [PubMed]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071-8. [Crossref] [PubMed]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 2001;7:263-72. [Crossref] [PubMed]
- Moynahan ME, Chiu JW, Koller BH, et al. Brca1 controls homology-directed DNA repair. Mol Cell 1999;4:511-8. [Crossref] [PubMed]
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov 2015;5:1137-54. [Crossref] [PubMed]
- Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer 2004;4:814-9. [Crossref] [PubMed]
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008;26:3785-90. [Crossref] [PubMed]
- Keim R, Ferguson DJ. Contralateral recurrence and prognostic factors in familial non-BRCA1/2-associated breast cancer (Br J Surg 2006; 93: 961-968). Br J Surg 2007;94:121; author reply 121-2. [Crossref] [PubMed]
- Lee JM, Hays JL, Annunziata CM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst 2014;106:dju089. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- Sahin IH, Lowery MA, Stadler ZK, et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hepatol 2016;10:893-905. [Crossref]
- Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109-13. [Crossref] [PubMed]
- Connor AA, Gallinger S. Hereditary Pancreatic Cancer Syndromes. Surg Oncol Clin N Am 2015;24:733-64. [Crossref] [PubMed]
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005-9. [Crossref] [PubMed]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1999;91:1310-6. [Crossref] [PubMed]
- Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res 2002;62:3789-93. [PubMed]
- Lucas AL, Frado LE, Hwang C, et al. BRCA1 and BRCA2 germline mutations are frequently demonstrated in both high-risk pancreatic cancer screening and pancreatic cancer cohorts. Cancer 2014;120:1960-7. [Crossref] [PubMed]
- Salo-Mullen EE, O'Reilly EM, Kelsen DP, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015;121:4382-8. [Crossref] [PubMed]
- Holter S, Borgida A, Dodd A, et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol 2015;33:3124-9. [Crossref] [PubMed]
- Hu C, Hart SN, Bamlet WR, et al. Prevalence of Pathogenic Mutations in Cancer Predisposition Genes among Pancreatic Cancer Patients. Cancer Epidemiol Biomarkers Prev 2016;25:207-11. [Crossref] [PubMed]
- Roberts NJ, Norris AL, Petersen GM, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov 2016;6:166-75. [Crossref] [PubMed]
- Grant RC, Selander I, Connor AA, et al. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology 2015;148:556-64. [Crossref] [PubMed]
- Geurts J, Evans DB, Tsai S. Genetic screening for patients with pancreatic cancer: Frequency of high-risk mutations. J Clin Oncol 2015;33:e12526. (Meeting Abstracts).
- Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17:569-77. [Crossref] [PubMed]
- Blanco A, de la Hoya M, Osorio A, et al. Analysis of PALB2 gene in BRCA1/BRCA2 negative Spanish hereditary breast/ovarian cancer families with pancreatic cancer cases. PLoS One 2013;8:e67538. [Crossref] [PubMed]
- Harinck F, Kluijt I, van Mil SE, et al. Routine testing for PALB2 mutations in familial pancreatic cancer families and breast cancer families with pancreatic cancer is not indicated. Eur J Hum Genet 2012;20:577-9. [Crossref] [PubMed]
- Peterlongo P, Catucci I, Pasquini G, et al. PALB2 germline mutations in familial breast cancer cases with personal and family history of pancreatic cancer. Breast Cancer Res Treat 2011;126:825-8. [Crossref] [PubMed]
- van der Heijden MS, Yeo CJ, Hruban RH, et al. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res 2003;63:2585-8. [PubMed]
- Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res 2005;65:383-6. [PubMed]
- Rogers CD, van der Heijden MS, Brune K, et al. The genetics of FANCC and FANCG in familial pancreatic cancer. Cancer Biol Ther 2004;3:167-9. [Crossref] [PubMed]
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 2015;121:25-33. [Crossref] [PubMed]
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 2014;32:2001-9. [Crossref] [PubMed]
- Maxwell KN, Wubbenhorst B, D'Andrea K, et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med 2015;17:630-8. [Crossref] [PubMed]
- Futreal PA, Liu Q, Shattuck-Eidens D, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science 1994;266:120-2. [Crossref] [PubMed]
- Lancaster JM, Wooster R, Mangion J, et al. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet 1996;13:238-40. [Crossref] [PubMed]
- Birgisdottir V, Stefansson OA, Bodvarsdottir SK, et al. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res 2006;8:R38. [Crossref] [PubMed]
- Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol 2010;28:3570-6. [Crossref] [PubMed]
- Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011;306:1557-65. [Crossref] [PubMed]
- Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215-28. [Crossref] [PubMed]
- Chantrill LA, Nagrial AM, Watson C, et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res 2015;21:2029-37. [Crossref] [PubMed]
- Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399-405. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744. [Crossref] [PubMed]
- Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 2015;21:211-20. [Crossref] [PubMed]
- Synowiec A, Wcisło G, Bodnar L, et al. Clinical features and outcomes of germline mutation BRCA1-linked versus sporadic ovarian cancer patients. Hered Cancer Clin Pract 2016;14:1. [Crossref] [PubMed]
- Zhang N, Ouyang TH, Zhou Q, et al. Prodynorphin gene promoter polymorphism and temporal lobe epilepsy: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2015;35:635-9. [Crossref] [PubMed]
- Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132-8. [Crossref] [PubMed]
- Golan T, Sella T, Javle MM, et al. Overall survival and clinical characteristics in BRCA mutation carriers with stage I/II pancreatic cancer(PC). J Clin Oncol 2015;33:287. (Meeting Abstracts). [PubMed]
- Kim H, Saka B, Knight S, et al. Having pancreatic cancer with tumoral loss of ATM and normal TP53 protein expression is associated with a poorer prognosis. Clin Cancer Res 2014;20:1865-72. [Crossref] [PubMed]
- Okazaki T, Jiao L, Chang P, et al. Single-nucleotide polymorphisms of DNA damage response genes are associated with overall survival in patients with pancreatic cancer. Clin Cancer Res 2008;14:2042-8. [Crossref] [PubMed]
- Li D, Li Y, Jiao L, et al. Effects of base excision repair gene polymorphisms on pancreatic cancer survival. Int J Cancer 2007;120:1748-54. [Crossref] [PubMed]
- Li D, Frazier M, Evans DB, et al. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. J Clin Oncol 2006;24:1720-8. [Crossref] [PubMed]
- Li D, Liu H, Jiao L, et al. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Res 2006;66:3323-30. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Lohse I, Borgida A, Cao P, et al. BRCA1 and BRCA2 mutations sensitize to chemotherapy in patient-derived pancreatic cancer xenografts. Br J Cancer 2015;113:425-32. [Crossref] [PubMed]
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 2012;30:2654-63. [Crossref] [PubMed]
- Tan DS, Rothermundt C, Thomas K, et al. "BRCAness" syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol 2008;26:5530-6. [Crossref] [PubMed]
- Arun B, Bayraktar S, Liu DD, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 2011;29:3739-46. [Crossref] [PubMed]
- Sonnenblick A, Kadouri L, Appelbaum L, et al. Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy. Cancer Biol Ther 2011;12:165-8. [Crossref] [PubMed]
- Chalasani P, Kurtin S, Dragovich T. Response to a third-line mitomycin C (MMC)-based chemotherapy in a patient with metastatic pancreatic adenocarcinoma carrying germline BRCA2 mutation. JOP 2008;9:305-8. [PubMed]
- James E, Waldron-Lynch MG, Saif MW. Prolonged survival in a patient with BRCA2 associated metastatic pancreatic cancer after exposure to camptothecin: a case report and review of literature. Anti-cancer drugs 2009;20:634-8. [Crossref] [PubMed]
- Lowery M, Shah MA, Smyth E, et al. A 67-year-old woman with BRCA 1 mutation associated with pancreatic adenocarcinoma. J Gastrointest Cancer 2011;42:160-4. [Crossref] [PubMed]
- Vyas O, Leung K, Ledbetter L, et al. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs 2015;26:224-6. [Crossref] [PubMed]
- Lowery MA, Lee A, Tobias E, et al. Evaluation of PARP inhibition as a platinum sparing strategy in Brca2-deficient pancreatic tumors. J Clin Oncol 2014;32:e15237. (Meeting Abstracts).
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Bendell J, O'Reilly EM, Middleton MR, et al. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann Oncol 2015;26:804-11. [Crossref] [PubMed]
- Lowery MA, Kelsen DP, Smith SC, et al. Phase II trial of veliparib (V) in patients (pts) with previously treated BRCA or PALB2-mutated (mut)pancreas adenocarcinoma (PC). J Clin Oncol 2015;33:358. (Meeting Abstracts).
- O'Reilly EM, Lowery MA, Segal MF, et al. Phase IB trial of cisplatin (C), gemcitabine (G), and veliparib (V) in patients with known or potential BRCA or PALB2-mutated pancreas adenocarcinoma (PC). J Clin Oncol 2014;32:abstr 4023.


