Cost-effectiveness of precision medicine in gastrointestinal stromal tumor and gastric adenocarcinoma
Introduction to precision medicine in gastric cancers
With an estimated 26,370 new diagnoses and 10,730 deaths in the US in 2016, gastric adenocarcinoma (GA) remains an uncommon, but deadly cancer within the Western world (1). Its associated high mortality is due to not only the tumor’s innate aggressiveness, but also to its late stage of presentation, with more than 60% of patients diagnosed with at least locally advanced disease. Despite recent advances in diagnosis and treatment, the 5-year overall survival (OS) remains poor at 29% (2). Meanwhile, gastrointestinal stromal tumors (GIST), with an estimated 4,000–6,000 new diagnoses in the US in 2016, is an extraordinarily rare, but highly curable cancer, with a 5-year OS ranging from 60–85% (1,3). As opposed to those with GA, the OS for patients with GIST, especially those with more advanced disease, has improved markedly over the past decade.
Within the past 15–20 years, with the incorporation of high output tumor mutational analyses and improved understanding regarding the mechanisms of cancer growth/metastasis, novel targets and their associated treatments have emerged within the field of oncology and are now regularly incorporated into the clinical care of patients in the US. Novel, more tumor-specific, non-chemotherapy agents, which include those that are commonly used in the treatment of patients with both GA and GIST, fall under a broader treatment strategy, termed “precision medicine” (4). The Precision Medicine Initiative (PMI), which called for the allocation of $215 million US dollars to the National Institutes of Health (NIH) and the National Cancer Institute (NCI), was recently unveiled by US President Barack Obama, and not only highlighted the successes and limitations of precision medicine as it pertained to past and current aspects of medical diagnosis and treatment, but also the value in its potential future utility with regard to improving cancer care in the US and decreasing cancer-specific patient mortality (5).
Gastric adenocarcinoma (GA)
Through the use of precision medicine-associated diagnostic testing, 7–22% of GA have been found to overexpress the human epidermal receptor 2 (HER2), a prerequisite for the use and beneficial effect of the HER2 targeting agent, trastuzumab (6-11). Based upon these initial studies and other early phase studies, a phase III trial was conducted to evaluate trastuzumab in HER2 positive (HER2+) metastatic GA (mGA). Patients randomized to receive trastuzumab, in conjunction with chemotherapy (cisplatin and capecitabine or 5-fluorouracil) and then as monotherapy thereafter, had a significantly improved OS compared to those who received chemotherapy alone (13.8 vs. 11.1 months; hazard ratio 0.74; 95% CI, 0.60–0.91; P=0.0046). The results of this study served as the impetus for the drug’s ensuing Food and Drug Administration (FDA) approval (9).
Other HER2 targeting agents have shown mixed results in GA. Pertuzumab has recently been FDA approved for use in select patients with HER2 positive breast cancer and has demonstrated considerable synergistic activity with trastuzumab (12). Clinical trials determining its effectiveness are ongoing and include the phase III JACOB trial evaluating the combination of pertuzumab, trastuzumab, and chemotherapy in mGA and the phase III PETRARCA trial comparing standard combination chemotherapy with trastuzumab and pertuzumab with combination chemotherapy for neoadjuvant use in locally advanced GA (13). Meanwhile, ado-trastuzumab emtansine (T-DM1), another recently approved HER2 targeted agents used and shown to be very effective for select patients with breast cancer has also been evaluated in GA (14). The phase III GATSBY trial compared T-DM1 with taxane chemotherapy in advanced gastric cancer, but a preliminary analysis revealed that the study failed to meet their primary endpoint (15). Lapatinib, an agent that targets both epidermal growth factor receptor (EGFR) and HER2, has been shown to have clinical benefit in select HER2 positive breast cancer patients (16). However, two phase III trials have evaluated its use in the 1st (LOGIC) and 2nd line setting (TyTAN) when combined with standard chemotherapy and have found no benefit (17).
Similar to other solid tumors, the uncontrolled growth characterized by GA is highly dependent upon local blood supply and angiogenesis. With this in mind, the anti-angiogenic agent ramucirumab, which primarily targets an angiogenesis mediator vascular endothelial growth factor 2 (VEGFR2), was shown to marginally, but significantly improve OS in mGA patients (5.2 vs. 3.8 months; hazard ratio 0.78; 95% CI, 0.60–0.99; P=0.047). Subsequently, the FDA approved the drug for the use of patients with mGA, either as monotherapy, or in combination with paclitaxel chemotherapy (18).
Gastrointestinal stromal tumors (GISTs)
Mutational analysis, with regard to GIST, have found that mutations tend to be mutually exclusive, and that 80% have protein coding (KIT) gene mutations that lead to the activation of a targetable KIT receptor (19-21). While nearly 75% of these mutations affect exon 11, they can also affect exon 9, 13, or 17 (22,23). Approximately 7% of GISTs harbor mutations in the tyrosine kinase platelet derived growth factor receptor (PDGFRA), and even less commonly, have only an inactivation of the succinate dehydrogenase complex (24,25). The discovery and in-depth characterization of the mutations have not only led to the use of precision medicine for GIST, but also reemphasized an important concept of precision medicine: different targetable mutations have varying responses to different drugs, and specific mutations can dictate the minimal effective treatment dose. Imatinib, a tyrosine kinase inhibitor (TKI) that is historically known for its revolutionary impact in the treatment of Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia through the inhibition of the BCR-ABL gene product, has been found to also inhibit c-KIT and PDGFA. As a result of the improved relapse free survival (RFS) seen in a recent phase III trial among patients with high-risk resected GIST who received 36 versus 12 months (the previous approved treatment duration) of adjuvant therapy, the FDA updated the drug’s prior approval for this indication (26). Although imatinib was initially FDA approved in the unresectable/metastatic setting based upon improved response rates compared with systemic chemotherapy, it was subsequently shown to also improve OS when used for this indication (27). Sunitinib, another multi-targeted TKI, through its inhibition of PDGFR, was found in a phase III trial to be effective and improve outcomes among unresectable/metastatic GIST patients who were intolerant or refractory to imatinib (28). Finally, regorafenib, another multi-targeted TKI, through its inhibition of KIT and PDGFR, was shown in a phase III trial to improve outcomes among unresectable/metastatic GIST patients who were refractory to both imatinib and sunitinib (29).
Precision medicine has become a national priority and has not only been incorporated into the care of patients with GA and GIST, but in some instances, has been shown to significantly improve outcomes. Despite its successes and potential future utility, precision medicine can be costly. In fact, a recent study determined that the total yearly cost of cancer care in US was approximately $124.5 billion dollars, a number that was projected to increase to approximately $157.7 billion by 2020 (30). With the surge in innovation comes an important discussion regarding the cost, management, and sequencing of therapies, as well as the concern regarding the overall sustainability of the current health care system and the ability of public and private payers to cover increasing costs. In this paper, we will review the current literature regarding cost and cost-effectiveness associated with precision medicine in GA and GIST.
Drug prices vary between different countries
The price of oncology drugs varies considerably by country, as was shown in a recent study looking at the prices in 16 European countries, Australia, and New Zealand and found that the difference from the highest to lowest priced country varied between 28% and 388% (31). From a US perspective, the average price of cancer drugs for a year of therapy increased from $5,000 to $10,000 before 2000 to more than $100,000 by 2012 and although 85% of cancer basic research is funded through public payment, the US pays 50% to 100% more for the same drug compared to other countries (32). Given the paucity of cost effective analyses for each individual country, the study relies on international data and thus caution must be taken when attempting to extrapolate cost analyses from one country to another.
Gastric/esophagogastric adenocarcinomas (GA)
Biomarker testing
Similar to its use in breast cancer, HER2 testing typically involves initial tumor testing with immunohistochemistry (IHC), with grading scores ranging from 0–3+. If a tumor is found to have 0 or 1+ HER2 expression, they are deemed to have HER2 negative GA and no further testing is performed. Conversely, if the tumor is found to have 3+ HER2 expression, tumors are considered HER2+ and no additional testing is performed. In cases where tumors are deemed to have 2+ HER2 expression, or there is a question regarding the accuracy of a tumor with 0 or 1+ expression, fluorescent in-situ hybridization (FISH) (via chromogenic or silver in-situ hybridization) using either HER2 copy number or HER2/chromosome 17 ratio (HER2/CEP17) (33) is performed for confirmation. If the tumor is found to have an average HER2 copy number ≥6.0 signals/cell and/or a HER2/CEP17 ratio of 2 or greater, the tumor is considered HER2+. Trastuzumab has been found to improve OS most significantly among patients with IHC 3+ tumors, compared with patients with IHC 2+, FISH positive tumors where it is less effective, and IHC 0 or 1+ tumors where it has been found to be ineffective (9). Although the technique has yet to be widely adopted secondary to availability and cost, there is some evolving research in the utility of a reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) assay that not only measures relative HER2 mRNA levels, but has been shown to be highly concordant with the other, more widely used methods of detecting HER2 expression (34).
There have been several concerns related to HER2 testing in GA. HER2 protein expression in GA, as opposed to similar testing in breast cancer, tends to spare the digestive luminal membrane, and thus results in a greater false positive rate. Similar to HER2 testing done in other cancer types, GA intra-tumor HER2 heterogeneity increases the risk for false positives and false negatives, which have been reported to be as high as 17% (35).
Trastuzumab
The National Institute for Health and Care Excellence (NICE) is a British governmental agency that was established in its current state in 2005. Based upon cost-effectiveness analyses, they publish guidelines related to health technologies, clinical practice, public health, and social services within the National Health Service (NHS; new and existing) (36). These guidelines are used to ultimately decide whether or not a health technology, such as a new drug, can be used in clinical practice within the United Kingdom (UK). In response to public pressure due to the UK Prime Minister David Cameron introduced the Cancer Drugs Fund (CDF) in 2011 to fund drugs that were not approved by NICE. Although the fund initially called for a budget of $370 million/year, during 2014–2015, the NHS reported going over budget by over $100 million and would have to subsequently cut funding for more than 20 oncology drugs (37,38). Using data from the TOGA trial (9), NICE estimated the incremental cost effectiveness ratio (ICER) of trastuzumab to be between $49,011–$54,457, and subsequently appraised its use for treatment naïve mGA patients, whose tumors were deemed to be HER2+ as defined by an IHC 3+ result (39,40) (Table 1). This appraisal was in contrast to their previous decision for the same indication, but which also included those patients with HER2 IHC 2+/FISH positive, for which trastuzumab was determined to have an ICER between $73,006 and $108,747 (42). A Japanese study reported similar findings to NICE, and found that tumors from patients which were IHC 2+/FISH+ were associated with an ICER of $90,440/quality adjusted life years (QALY) and $65,379/life year gained (LY), whereas patients with tumors that were deemed IHC 3+ had an associated ICER of $59,930/QALY and $42,496/LY (43). A study examined the trastuzumab prescribing impact in a large teaching hospital in Ireland, and found that the total treatment related cost of trastuzumab was $26,152/patient, with a total cost per year (1 teaching hospital) of $287,668, and a total cost per year (country of Ireland) of $915,306 (44).
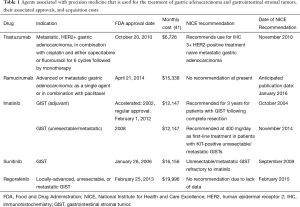
Full table
Health utility values, elicited through the use of well-established, preference-based measures, such as the EuroQol-5 Dimension (EQ-5D), serve a critical role in cost-effectiveness studies as they are able to quantify particular health states that are encountered among cancer patients. A recent review article found that the health utility value of newly diagnosed mGA fell somewhere between 0.66–0.73, but found that these health states dropped precipitously (disutility: −0.20–0.50) with multiple treatments, multiple progressions, and disease/treatment related side effects affecting quality of life (QOL), most notably dysphagia and weight loss (45).
Ramucirumab
Although there is a paucity of data looking at the cost-effectiveness of ramucirumab in mGA, one recent review questioned the likelihood of its cost-effectiveness given the results of the phase III trial REGARD, which compared it to placebo and led to the drug’s FDA approval (median OS: 5.2 vs. 3.8 months) (46,47). Although an official guideline is scheduled to be released in January of 2016, the NICE cost-effectiveness analysis concluded that the most plausible ICER for ramucirumab plus paclitaxel, compared with best supportive care (BSC) plus paclitaxel, for patients with mGA was $443,386/QALY gained, and $204,314/QALY gained for ramucirumab monotherapy when compared with BSC (48). Although the study looked at ramucirumab as it is used in metastatic colorectal cancer (mCRC), the OS improvement reported in the phase III mCRC RAISE trial (1.6 months) was similar to that seen in the mGA REGARD trial (1.4 months). The monthly drug acquisition cost of ramucirumab was calculated to be $15,338, which was significantly greater than that of bevacizumab and ziv-aflibercept, both of which were previously shown to not be cost-effective in mCRC (49,50). Despite these studies, the jury is still out on its cost-effectiveness in mGA and more data will need to be generated in order to properly evaluate ramucirumab in mGA.
Gastrointestinal stromal tumors (GISTs)
Biomarker testing
Most experts recommend a more detailed analysis regarding the KIT mutation in patients with unresectable/metastatic GIST, as it provides the patient and clinician with valuable prognostic and predictive information (51). For example, patients with exon 11 KIT mutations unresectable/metastatic GIST not only have a substantially greater imatinib treatment response, but also have an improved progression free survival (PFS) and OS, when compared to those patients with either exon 9 KIT mutations, or those without a detectable mutation in either KIT or PDGFRA (52). Dose-response trials have shown that, as opposed to tumors harboring exon 11 KIT mutation or those that are KIT wild-type, higher daily doses of imatinib were found to improve treatment response and PFS among those with exon 9 KIT mutations (52,53). As a result of these studies, the National Comprehensive Cancer Network (NCCN) recommended the use of imatinib at a starting dose of 800 mg daily for those patients with exon 9 KIT mutation GISTs (54). However, given the fact that some centers do not have access to a more detailed mutational analysis, many will employ a maneuver, in which they treat all patients starting at 400 mg daily and then upon tumor progression, will either increase the dose or switch to second-line therapy.
Studies have shown that different GIST PDGFRA mutations confer varying degrees of imatinib sensitivity (24,55). One large series of PDGFRA-mutant GISTs showed that 63% of patients had the imatinib-resistant substitution D842V (23). Comprehensive molecular analyses, which specify the type of PDGFRA GIST mutation, are currently not routinely carried out at all hospitals, but given promising recent evidence, may play a role in the future management of GIST. Currently, there are clinical trials underway that are evaluating the safety and efficacy of new TKIs that are specifically engineered for the treatment of tumors with the PDGFRA D824V mutation (56).
Mutational testing in GIST has been performed through a variety of methods, all of which have varying degrees of sensitivity and associated cost. One method, referred to denaturing high-pressure liquid chromatography (DHPLC), was found in a recent study to be less costly and labor-intensive and with comparable sensitivity, when compared with the most commonly used technique, direct polymerase chain reaction (PCR) sequencing (57). Another study investigated the utility of microfluidic deletion/insertion analysis as an initial GIST mutation screening strategy and found that although it only detected 75% of KIT mutated cases, it was associated with a significantly lower cost than both DHPLC and PCR and showed future promise as a screening tool (58). Other techniques that have been investigated as tools for GIST mutation detection have included PCR-single strand conformation polymorphism testing and length analysis of PCR products, both of which deliver very accurate and detailed results, but are associated with a considerable cost (59).
TKI therapy
Using the results of a phase III trial, a recent study evaluated the CE of 3-year of adjuvant imatinib versus 1-year of therapy (prior standard of care). With a total lifetime per-patient cost of $302,100 (3 years), compared to a total lifetime per-patient cost of $217,800 (1 year), the ICER was found to be $62,600/QALY, well within the commonly cited willingness to pay (WTP) thresholds for cost effective cancer therapy (60). A similar study, conducted from a European perspective, also found that 3 years of adjuvant imatinib therapy was cost-effective when compared to 1 year, with an even lower ICER of $32,619/QALY (61). Another model developed by Novartis before the drug price increase in 2012 found that that the ICER of adjuvant imatinib decreased over time: $56,251–$107,981 after 2 years, $29,844–$52745 after 5 years, and from $23,372–$37,100 after 10 years (62). Subsequently, based upon these and other studies, NICE issued an appraisal for imatinib for 3 years of adjuvant therapy (63). Researchers evaluated the budgetary impact of treatment with adjuvant imatinib for 1 year following surgical resection of KIT-mutated GIST, and found the net budgetary impact to be $0.01 per member per month in the third year after introduction, with 11.7–21.9% of the budgetary cost being offset by the reduction in costs associated with GIST recurrence (64).
Imatinib, when used in unresectable/metastatic GIST, has been shown to improve OS when compared to placebo (5.8 vs. 2.7 years). Using data from this phase III trial, a cost-effectiveness analysis was conducted and found that the ICER was $38,723/QALY (65). Another study conducted a cost-effectiveness analysis of second-line treatment in unresectable/metastatic GIST with high dose imatinib (800 mg PO daily), sunitinib, or BSC and found that high dose imatinib had a median cost of treatment of $35,225, whereas sunitinib and palliative care were associated with median costs of $17,805 and $2,071, respectively (66). Sunitinib, by delivering the greatest survival benefit (5.64 progression free months, 1.4 LYG), was found to be cost effective and fall below a WTP threshold of $50,000 in 38% of patients. Meanwhile, imatinib and BSC were both associated with a lower OS (5.28 vs. 2.58 progression free months, 1.31 and 1.08 LYG) and lower likelihood of being found to be cost effective.
A similar study from China found the ICER to be $5,664/QALY when comparing sunitinib versus intermediate dose imatinib 600 mg and $19,554/QALY, when comparing treatment with sunitinib versus BSC (67). Look-Hong et al. created a Markov model that evaluated the costs associated with surgery in combination with imatinib or sunitinib in seven different scenarios, which varied by type of TKI, TKI dose, and disease status. They found that the most inexpensive scenario was no surgery and the most costly was surgery in patients with progressive disease plus treatment with imatinib 800 mg. Most of the costs incurred in the seven different scenarios were attributed to the TKI drug acquisition cost (68). Based upon these studies, NICE advised against the use of imatinib at a dose of 600 or 800 mg for patients with unresectable/metastatic GISTs whose disease had progressed after treatment with imatinib at a dose of 400 mg (69). Meanwhile, they approved the use of sunitinib for the same indication (70).
A study looked at the cost-effectiveness of regorafenib compared with BSC in unresectable/metastatic GIST and found the total costs of patients treated with regorafenib to be $28,283, compared with $21,136 for BSC, with an ICER of $32,760/QALY (71). A study from Turkey found that the total costs associated with regorafenib were $7,553 compared with $558 for BSC, yielding an ICER of $5,435/QALY (72). Given the lack of published high quality data, NICE has yet to issue a statement with regard to the use of regorafenib for patients with unresectable/metastatic GIST (73).
Conclusions and future directions
Future approvals
There are several emerging precision medicine-related diagnostic approaches and treatments in GA and GIST that have the possibility of coming to the forefront. Some of these treatments include the previously mentioned HER2 targeted agent pertuzumab, which when combined with trastuzumab and docetaxel in the first-line HER2 positive mBC cancer setting, was not a cost-effective strategy when compared to trastuzumab and docetaxel alone (74). However, a criticism of this analysis was that it failed to account for the sequential (as opposed to one time) drug prescribing practice that is commonly employed in patients with metastatic disease (75). Another emerging treatment option is immune checkpoint inhibitor, pembrolizumab, which acts through the inhibition of programmed death 1 (PD1) and was recently shown in a phase II trial to have an impressive overall response rate in several solid tumors. If this agent were to be FDA approved in the future, not only would drug acquisition costs be a factor, but also its associated biomarker testing (IHC staining for programmed death ligand 1) (76). A phase II trial looking at the poly-ADP ribose polymerase (PARP) inhibitor olaparib showed encouraging activity in mGA, especially among patients with low ataxia telangiectasia (ATM) protein expression (77). Therefore, once again not only would a future approval bring the CE of the drug into question, but also the aforementioned biomarker. In regards to unresectable/metastatic GIST, other TKI’s, such as sorafenib, dasatinib, nilotinib, ponatinib, and pazopanib, have been used in early phase trials and show some promise. If they are able to demonstrate favorable phase III results in the future, all of these agents have the possibility of being FDA approved, especially if they are shown to have activity in KIT wild-type GIST, where TKIs only produce modest response rates (78-80). All of these TKIs have been shown to have significant acquisition costs and treatment related costs related to rare but serious adverse effects.
Framework of cost-effectiveness studies and their impact on healthcare policy in both the US and internationally
Over the past 10–15 years, novel treatments have emerged for cancers such as GA and GIST. While diagnostic testing and associated treatments in GA are expensive and produce only marginal benefit, those associated with GIST, despite being costly, produce significant improvements in patient outcomes. Despite the significant difference in impact, the agents associated with these cancers have similar acquisition costs. Currently, the cost-effectiveness of a drug or biomarker has no impact on its FDA approval, and once approved, public and private payers typically have to reimburse manufacturers without negotiation. In fact, the Patient Protection and Affordable Care Act of 2010 prohibited Centers for Medicare & Medicaid Services (CMS) from using cost-effectiveness as a factor in making reimbursement and coverage decisions about health care services and products (81). With the refusal to acknowledge the importance of regulation and value-based health care pricing, the US now leads all major countries in health care spending [17.5% of the US gross domestic product (GDP); $618.7 billion CMS spending in 2014], which most notably includes drug acquisition, procedure, and hospitalization costs. Given the significant spending, the strained healthcare system has created an unsustainable predicament for the US economy.
Despite its healthcare spending, the US consistently ranks near the middle of the pack among developed nations in healthcare quality and efficiency (includes measures such as life expectancy and cancer-related mortality) (82). As precision medicine in GA and GIST continues to evolve, the importance of value-based medicine has become even more paramount.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- SEER Stat Fact Sheets: Stomach Cancer. Available online: http://seer.cancer.gov/statfacts/html/stomach.html. Accessed January 5th, 2016.
- Call J, Walentas CD, Eickhoff JC, et al. Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer 2012;12:90. [Crossref] [PubMed]
- Park YS, Hwang HS, Park HJ, et al. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: which scoring system should we use? Hum Pathol 2012;43:413-22. [Crossref] [PubMed]
- President Obama Announces Plans for New Precision Medicine Initiative. American Association for Cancer Research. Available online: http://www.aacr.org/AdvocacyPolicy/GovernmentAffairs/Pages/CPM150210-president-obama-announces-plans-for-new-precision-medicine-initiative.aspx#.Vo7DgBUrLWI
- Barros-Silva JD, Leitão D, Afonso L, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer 2009;100:487-93. [Crossref] [PubMed]
- Takehana T, Kunitomo K, Kono K, et al. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer 2002;98:833-7. [Crossref] [PubMed]
- Liang Z, Zeng X, Gao J, et al. Analysis of EGFR, HER2, and TOP2A gene status and chromosomal polysomy in gastric adenocarcinoma from Chinese patients. BMC Cancer 2008;8:363. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol 2011;18:2833-40. [Crossref] [PubMed]
- Koopman T, Smits MM, Louwen M, et al. HER2 positivity in gastric and esophageal adenocarcinoma: clinicopathological analysis and comparison. J Cancer Res Clin Oncol 2015;141:1343-51. [Crossref] [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- “Pertuzumab” and “Gastric”. Clinicaltrials.gov. U.S. National Institutes of Health. Available online: https://clinicaltrials.gov/ct2/results?term=pertuzumab+gastric&Search=Search
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Ado-trastuzumab Emtansine Fails Phase II/III Gatsby Trial. ADC Review/Journal of Antibody-drug Conjugate. Available online: http://adcreview.com/news/ado-trastuzumab-emtansine-fails-phase-iiiii-gatsby-trial
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [Crossref] [PubMed]
- Janjigian YY. Lapatinib in Gastric Cancer: What Is the LOGiCal Next Step? J Clin Oncol 2016;34:401-3. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000;156:791-5. [Crossref] [PubMed]
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118-21. [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 2003;125:660-7. [Crossref] [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [Crossref] [PubMed]
- Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A 2011;108:314-8. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117-28. [Crossref] [PubMed]
- Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol 2016;17:39-47. [Crossref] [PubMed]
- Kantarjian H, Rajkumar SV. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc 2015;90:500-4. [Crossref] [PubMed]
- Fox SB, Kumarasinghe MP, Armes JE, et al. Gastric HER2 Testing Study (GaTHER): an evaluation of gastric/gastroesophageal junction cancer testing accuracy in Australia. Am J Surg Pathol 2012;36:577-82. [Crossref] [PubMed]
- Park T, Griggs SK, Suh DC. Cost Effectiveness of Monoclonal Antibody Therapy for Rare Diseases: A Systematic Review. BioDrugs 2015;29:259-74. [Crossref] [PubMed]
- Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [Crossref] [PubMed]
- Who we are. National Institute for Health and Care Excellence (NICE). Available online: https://www.nice.org.uk/about/who-we-are
- Cancer Drugs Fund. Cancer Research UK. Available online: http://www.cancerresearchuk.org/about-cancer/cancers-in-general/cancer-questions/cancer-drugs
- Cancer drugs fund cuts 23 treatments. BBC News. Available online: http://www.bbc.com/news/health-34153136
- Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 2010;457:299-307. [Crossref] [PubMed]
- Spackman E, Rice S, Norman G, Suh DC, et al. Trastuzumab for the treatment of HER2-positive metastatic gastric cancer: a NICE single technology appraisal. Pharmacoeconomics 2013;31:185-94. [Crossref] [PubMed]
- 2016 ASP Drug Pricing Files. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2016ASPFiles.html
- Holden J, Garrett Z, Stevens A. NICE guidance on trastuzumab for the treatment of HER2-positive metastatic gastric cancer. Lancet Oncol 2011;12:16-7. [Crossref] [PubMed]
- Shiroiwa T, Fukuda T, Shimozuma K. Cost-effectiveness analysis of trastuzumab to treat HER2-positive advanced gastric cancer based on the randomised ToGA trial. Br J Cancer 2011;105:1273-8. [Crossref] [PubMed]
- Collins IM, King F, O’Byrne K. Cost impact of trastuzumab prescribing in the treatment of advanced Her2 positive gastric cancer in Ireland. Ir J Med Sci 2012;181:451-2. [Crossref] [PubMed]
- Carter GC, King DT, Hess LM, et al. Health state utility values associated with advanced gastric, oesophageal, or gastro-oesophageal junction adenocarcinoma: a systematic review. J Med Econ 2015;18:954-66. [Crossref] [PubMed]
- Wadhwa R, Elimova E, Shiozaki H, et al. Anti-angiogenic agent ramucirumab: meaningful or marginal? Expert Rev Anticancer Ther 2014;14:367-79. [Crossref] [PubMed]
- Gastric cancer (metastatic) - ramucirumab (after chemotherapy) [ID741]. National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/GID-TAG500/documents/html-content
- Goldstein DA, El-Rayes BF. Considering Efficacy and Cost, Where Does Ramucirumab Fit in the Management of Metastatic Colorectal Cancer? Oncologist 2015;20:981-2. [Crossref] [PubMed]
- Goldstein DA, Ahmad BB, Chen Q, et al. Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. J Clin Oncol 2015;33:3727-32. [Crossref] [PubMed]
- Goldstein DA, Chen Q, Ayer T, et al. First- and Second-Line Bevacizumab in Addition to Chemotherapy for Metastatic Colorectal Cancer: A United States-Based Cost-Effectiveness Analysis. J Clin Oncol 2015;33:1112-8. [Crossref] [PubMed]
- Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:64-7. [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [Crossref] [PubMed]
- GIST. NCCN Clinical Practice Guidelines in Oncology. Available online: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26:5360-7. [Crossref] [PubMed]
- Phase II Study of Crenolanib (CP-868,596), for the Treatment of Patients With Advanced Gastrointestinal Stromal Tumors With the D842-related Mutations and Deletions in the PDGFRA Gene. Available online: https://clinicaltrials.gov/ct2/show/NCT01243346?term=GIST+and+D842V+mutation&rank=1
- Zamò A, Bertolaso A, Franceschetti I, et al. Microfluidic Deletion/Insertion Analysis for Rapid Screening of KIT and PDGFRA Mutations in CD117-Positive Gastrointestinal Stromal Tumors. J Mol Diagn 2007;9:151-7. [Crossref] [PubMed]
- Battochio A, Mohammed S, Winthrop D, et al. Detection of c- KIT and PDGFRA Gene Mutations in Gastrointestinal Stromal Tumors. Am J Clin Pathol 2010;133:149-55. [Crossref] [PubMed]
- Emile JF, Lemoine A, Bienfait N, et al. Length analysis of polymerase chain reaction products: a sensitive and reliable technique for the detection of mutations in KIT exon 11 in gastrointestinal stromal tumors. Diagn Mol Pathol 2002;11:107-12. [Crossref] [PubMed]
- Sanon M, Taylor DC, Parthan A, et al. Cost-effectiveness of 3-years of adjuvant imatinib in gastrointestinal stromal tumors (GIST) in the United States. J Med Econ 2013;16:150-9. [Crossref] [PubMed]
- Majer IM, Gelderblom H, van den Hout WB, et al. Cost-effectiveness of 3-year vs 1-year adjuvant therapy with imatinib in patients with high risk of gastrointestinal stromal tumour recurrence in the Netherlands; a modelling study alongside the SSGXVIII/AIO trial. J Med Econ 2013;16:1106-19. [Crossref] [PubMed]
- Wilson J, Connock M, Song F, et al. Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess 2005;9:1-142. [Crossref] [PubMed]
- Imatinib for the adjuvant treatment of gastrointestinal stromal tumours. NICE technology appraisal guidance [TA326] Published date: November 2014. Available online: https://www.nice.org.uk/guidance/ta326
- Rubin JL, Taylor DC, Sanon M, et al. Budgetary impact of treatment with adjuvant imatinib for 1 year following surgical resection of kit-positive localized gastrointestinal stromal tumors. J Manag Care Pharm 2010;16:482-91. [Crossref] [PubMed]
- Contreras-Hernández I, Mould-Quevedo JF, Silva A, et al. A pharmaco-economic analysis of second-line treatment with imatinib or sunitinib in patients with advanced gastrointestinal stromal tumours. Br J Cancer 2008;98:1762-8. [Crossref] [PubMed]
- Ren H, Zhang J, Dong P. Cost-Effectiveness Of Sunitinib As Second-Line Treatment For Gastrointestinal Stromal Tumor (GIST) In China. Value Health 2015;18:A455. [Crossref] [PubMed]
- Look Hong NJ, Chang SL, Raut CP. The economic impact of cytoreductive surgery and tyrosine kinase inhibitor therapy in the treatment of advanced gastrointestinal stromal tumours: a Markov chain decision analysis. Eur J Cancer 2014;50:397-405. [Crossref] [PubMed]
- Imatinib for the treatment of unresectable and/or metastatic gastrointestinal stromal tumours. NICE technology appraisal guidance [TA209] Published date: November 2010. Available online: https://www.nice.org.uk/guidance/ta209
- Sunitinib for the treatment of gastrointestinal stromal tumours. NICE technology appraisal guidance [TA179] Published date: September 2009. Available online: https://www.nice.org.uk/guidance/ta179
- Sanz-Granda Á, Hidalgo-Figueruela F, Granell M. Estimation of the treshold price of regorafenib in the treatment of unresectable and/or metastatic gastrointestinal stromal tumors after failure on imatinib and sunitinib in Spain: cost-utility analysis. Value Health 2015;18:A464. [Crossref] [PubMed]
- Deger C, Telli F, Gunaldi M, et al. The cost-effectiveness of regorafenib in the treatment of metastatic/inoperable gastrointestinal stromal tumors in Turkey. Value Health 2015;18:A455. [Crossref] [PubMed]
- Regorafenib for metastatic colorectal cancer after treatment for metastatic disease (terminated appraisal). NICE technology appraisal guidance [TA334] Published date: February 2015. Available online: https://www.nice.org.uk/guidance/ta334
- Muro K, Bang YJ, Shankaran V, et al. Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab in KEYNOTE-012. J Clin Oncol 2015;33:abstr 3.
- Bang YJ, Im SA, Lee KW, et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J Clin Oncol 2015;33:3858-65. [Crossref] [PubMed]
- Trent JC, et al. A phase II study of dasatanib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol 2011;29:abstr 10006.
- Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs 2012;30:2377-83. [Crossref] [PubMed]
- Sawaki A, Nishida T, Doi T, et al. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer 2011;117:4633-41. [Crossref] [PubMed]
- Ganjoo KN, Villalobos VM, Kamaya A, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol 2014;25:236-40. [Crossref] [PubMed]
- Garber AM, Sox HC. The role of costs in comparative effectiveness research. Health Aff (Millwood) 2010;29:1805-11. [Crossref] [PubMed]
- Durkee BY, Qian Y, Pollom EL, et al. Cost-Effectiveness of Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2016;34:902-9. [Crossref] [PubMed]
- Diaby V, Adunlin G, Ali A, et al. Cost-effectiveness of treatment sequencing of Pertuzumab followed by T-DM1 in metastatic HER2-positive breast cancer. JAMA 2016. In Press.
- National expenditure data. Centers for Medicare & Medicaid Services. Available online: https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html


