Comparative effectiveness of chemopreventive interventions for colorectal cancer: protocol for a systematic review and network meta-analysis of randomised controlled trials
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, with approximately 1.36 million new cases in 2012 (1); it is the fourth leading cause of cancer death worldwide (1). The lifetime probability of a CRC diagnosis in the average-risk individual, defined as without personal history or family history of CRC and above the age of 50, is 5–6% (2,3). This increases up to 20% when there is an association of family history, and reaches a lifetime risk of 80–100% in hereditary CRC syndromes, such as in hereditary nonpolyposis colorectal cancer (HNPCC) and familial adenomatous polyposis (FAP), respectively (2). Mortality due to CRC is increasing owing to the late stage at which many cases present (3). Attention is therefore focusing on preventative strategies. Early detection and removal of pre-cancerous colorectal adenomas by screening, followed by appropriate therapy and continued surveillance, can decrease mortality (4). Despite evidence of the effectiveness of several screening methods (4), uptake of screening by any method continues to be low (5). Moreover, adoption of screening recommendations requires a large amount of health care resources; its success will also depend on a high compliance rate and regular follow-up. Therefore, there is increased focus on the potential use of chemoprevention as a complement to, or instead of, screening in the prevention of CRC.
Chemoprevention comprises the use of drugs or natural compounds to prevent the development of benign or malignant tumours (6). It is believed that the majority of CRCs develop from benign precursor lesions, the so-called adenomatous polyps or adenomas, through a series of genetic changes (adenoma-carcinoma sequence) during a time interval of at least 5 to 10 years (7,8). Mechanisms underlying chemoprevention include modulation of signalling cascades, and/or of the expression of genes involved in the regulation of cell proliferation, differentiation, and apoptosis, as well as the suppression of chronic inflammation, metastasis, and angiogenesis in adenoma-carcinoma sequence (9,10). Systematic reviews have shown that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) [cyclo-oxygenase-2 (COX-2) inhibitors] reduce the risk of developing colorectal adenomas and cancer (11-14). Similarly calcium and/or vitamin D (15,16), folic acid (17-19) and some anti-oxidants such as selenium (20-22) have also been linked to a possible decreased incidence of CRC. This has led to the concept of using such agents for the chemoprevention of CRC.
Despite evidence of the effectiveness of NSAIDs in preventing CRC, it was concluded that their benefits are outweighed by harms in average risk individuals, including those with a family history of CRC (23,24). However, their use continues to be recommended for high risk individuals with FAP, hereditary nonpolyposis colon cancer syndromes (Lynch I or II), or a history of CRC or adenomas (23,24). The use of other agents such as calcium, vitamin D and folic acid in preventing CRC is not clearly acknowledged in any clinical guidelines. Therefore, evidence of the comparative advantages of various agents for different risk individuals is required to guide clinical decision-making for the chemoprevention of CRC. There have been at least 22 systematic reviews addressing chemoprevention for CRC (11-14,20,25-42); however, no reviews have compared the effectiveness of different chemopreventive agents.
Considerable data are available for clinical outcomes for each chemopreventive agent; moreover, there are validated methodologies to indirectly compare their effectiveness (43-45). Therefore, we will use network meta-analysis (NMA) to perform a comprehensive comparative appraisal of the effects of agents used in the chemoprevention of CRC.
The objective of this study is to conduct a systematic review and NMA to identify the comparative effectiveness of agents for the prevention of CRC in average-high risk individuals.
Methods and analysis
Design
A systematic review of the clinical effectiveness of drug or natural product interventions for the prevention of CRC and/or adenomatous polyps will be undertaken according to the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA-P) statements (46).
Data sources
We will identify relevant randomised controlled trials (RCTs), by a systematic search of MEDLINE 1946 to Present (Via Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Via Ovid), Embase 1974 to present (Via Ovid) Cochrane CENTRAL Register of Controlled Trials 1991 to present (Via Ovid), CINAHL plus, International Pharmaceutical Abstracts and clinicaltrials.gov website. To identify comprehensive studies not captured by database searches, we will manually check the reference lists of published systematic reviews and articles retrieved after title and abstract exclusion.
Search strategies
A search of human studies in these databases from inception through to August/September, 2015 will be performed by using subject headings and free text terms. Three sets of search terms will be combined: terms for CRC or adenomatous polyps and terms for the relevant interventions and a search filter to identify RCTs. For all interventions, search will restrict to studies published from 2008 onwards because studies published up to 2007 could be identified from the published systematic reviews (12-14,20,28-30). The MEDLINE search strategy is shown in Supplementary 1; the same search strategy will be used for EMBASE and Cochrane CENTRAL Register of Controlled Trials (via OVID). We will use the appropriate keywords from MEDLINE search strategy to identify studies from CINAHL plus, International Pharmaceutical Abstracts and clinicaltrials.gov website.
Eligibility criteria
The retrieved reports will be screened according to the checklist of eligibility (see online Supplementary 2) and the eligibility criteria shown below including participants, interventions, controls, outcomes, types of study and other criteria.
Participants
Inclusion—the use of chemopreventive interventions will be assessed in the following three defined populations (4,47): individuals with no increased risk for CRC (described in this assessment as ‘average risk individuals’); individuals at increased risk of CRC (described in this assessment as ‘increased risk individuals’) because of history of adenomatous polyps, personal history of CRC, family history of CRC, or inflammatory bowel disease; individuals at high risk of CRC (described in this assessment as ‘high risk individuals’) because of FAP or Lynch syndrome/hereditary nonpolyposis colorectal cancer (HNPCC). Exclusion—individuals diagnosed with CRC.
Interventions
Inclusion—any RCT that evaluates the efficacy of following interventions at any dose and frequency, alone or in combination: aspirin, non-aspirin NSAIDs (including COX-2 inhibitors), folic acid, calcium and/or vitamin D, antioxidants: vitamin A, vitamin C, vitamin E, selenium and beta-carotene. Exclusion—this study will exclude whole-food interventions (such as fruits, fibre, green tea, vegetables). Other agents such as curcumin, omega-3 fatty acids, and resveratrol will not be included, because there are only preliminary data relating to these substances. Rofecoxib and valdecoxib were withdrawn from the market (48); and will therefore be excluded.
Controls
Inclusion—any RCT that evaluates the efficacy of intervention of interest, placebo, no intervention or another intervention not included in this assessment. Exclusion—any RCT that evaluates the efficacy of whole-food interventions.
Outcomes
Inclusion—any RCT that includes outcome measures of incidence of CRC, incidence/recurrence of any adenoma or change in polyp burden (number or size). The secondary outcomes include the incidence of adverse events and compliance to the treatment or discontinuation rates. Exclusion—any RCT with outcome measures other than outcome of interest.
Types of study
Inclusion—only RCTs will be included. Exclusion—observational cohort and case-control studies, case reports, experimental studies and reviews will be excluded.
Other criteria
Other inclusion criteria—follow-up period or duration in RCTs are at least 1 year for incidence of CRC. There will be no restrictions by type of setting of studies. Other exclusion criteria—we will exclude RCTs from the same population (duplicate studies) and not reporting effect estimates or with insufficient information to compute effect estimates.
Study section
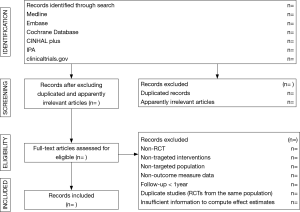
The titles and abstracts of literature search will be screened by one reviewer for potentially relevant studies according to the eligibility criteria (Supplementary 2) and double-checked by a second reviewer. After excluding the duplicated and apparently irrelevant studies, the remaining studies will be read in full text by two reviewers. We will document reasons for the exclusion of records during full-text screening. Disagreements between reviewers will be resolved by consensus. The primary selection process is presented in Figure 1.
Data extraction and quality assessment
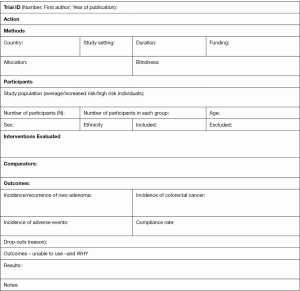
The following data will be extracted independently and in duplicate by two reviewers into a data extraction form (Supplementary 3): the name of the first author, year of publication, country in which the study was conducted, study setting, follow-up duration, patient’s characteristics (study population, number of participants in each group, age (mean/range), gender, and ethnicity), characteristics of interventions (interventions evaluated, dosage form, dose, frequency, length of treatment) and outcome measures (number of participants in whom a new adenoma occurred in the intervention and control groups, number of participants in whom CRC occurred in both groups, number of participants who developed adverse events related to interventions, compliance rate to interventions, drop-outs before the end of study and reasons for dropping out). Disagreement will be documented and resolved by discussion with the third reviewer; where there is still doubt, we will contact the authors for clarification.
Two reviewers will independently assess the risk of bias within each study by using a modified Cochrane risk of bias instrument (49,50). We will evaluate sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias (Table 1) (49,50). Reviewers will resolve disagreements by discussion, and one of two arbitrators will adjudicate unsolved disagreements.

Full table
Data synthesis
Quantitative synthesis or meta-analysis will be considered. If unsuitable due to the heterogeneity and/or small number of studies, a narrative overview of the findings of included studies will be presented with tabular summaries of extracted data. As an initial examination of the data, we will undertake traditional meta-analyses by combining evidence from direct, within-study comparisons between chemopreventive interventions. Heterogeneity between trials will be assessed by considering the I2 inconsistency statistic alongside the Chi2 (P>0.1). An I2 estimate greater than or equal to 50% accompanied by statistical significant Chi statistic, will be interpreted as evidence of substantial levels of heterogeneity (50). When substantial levels of heterogeneity are present, we will explore the underlying reasons. Publication bias will be assessed by using funnel plot asymmetry testing and Egger’s regression test. Direct meta-analysis will be performed using a random effects model to estimate effect size such as pooled relative risk (RR) and 95% confidence intervals (CI) incorporating within-and between-study heterogeneity (51). For continuous outcomes, weighted mean differences or standardized mean difference will be calculated, as appropriate, using the random-effects model and reported with 95% confidence intervals.
We will construct NMA to improve precision of the comparisons among chemopreventive interventions by combining direct and indirect evidence (43). Model with either consistency or inconsistency will be assessed in STATA (StataCorp LP, College Station, TX, USA) by contrasting direct and indirect estimates in each triangular loop by the methods described by Veroniki and colleagues (52).
The probability of each treatment being the best (lowest rate of CRC/adenomas) and/or safest (lowest rate of adverse events), the number-needed-to-treat [NNT; 95% credible interval (CrIs)], and the number-needed-to-harm (NNH; 95% CrIs) will be also calculated to provide measures of treatment efficacy. We will construct rankograms of cumulative rank probabilities of how each treatment ranks against each other in terms of being the 1st, 2nd, 3rd, and so forth, best treatment option. In addition, we will present a hierarchy of the efficacy and safety of the various chemopreventive interventions based on their cumulative rank probabilities and the Surface Area Under the Cumulative Rankograms (SUCRA, %) as proposed by Salanti and colleagues (45).
Quality of evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach will be used to rate the quality of evidence of estimates derived from NMA (53). Reviewers will, independently, assess the confidence in effect estimates for all outcomes using the following categories: risk of bias, inconsistency, indirectness, imprecision and publication bias (54). The confidence in estimates for each outcome will be categorized as follows: ‘high’ quality of evidence (we are very confident that the true effect lies close to that of the estimate of the effect); ‘moderate’ quality of evidence (we are moderately confident in the effect estimate and the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different); ‘low’ quality of evidence (our confidence in the effect estimate is limited and the true effect may be substantially different from the estimate of the effect); and ‘very low’ quality of evidence (we have very little confidence in the effect estimate and the true effect is likely to be substantially different from the estimate of effect) (53).
Subgroup analysis
Several subgroup analyses will be performed based on the follow-up period, characteristics of interventions (dosage form, dose and frequency), types of treatment (alone or in combination) and patients’ characteristics (age, sex).
Sensitivity analysis
We will assess the robustness of our results through sensitivity analysis by excluding trials at high risk of bias and using both fixed and random effects models.
Ethics and dissemination
Ethical issues
No ethical approval is required because this study includes no confidential personal data or interventions with the patients.
Publication plan
This protocol has been registered (registration number: CRD42015025849) with the PROSPERO (International Prospective Register of Systematic Reviews) (55). The procedures of this systematic review and NMA will be conducted in accordance with the PRISMA-compliant guideline. The results of this systematic review and NMA will be submitted to a peer-reviewed journal for publication.
Acknowledgements
The authors wish to thank Professor James Michael Menke, International Medical University, Malaysia and Professor Brian L Furman, Strathclyde Institute of Pharmacy and Biomedical Sciences, Glasgow, UK for their valuable comments and support which helped to improve the manuscript. The authors wish to thank Dr. Muhammad Radzi bin Abu Hassan, Head of Gastroenterology Service Ministry of Health, Malaysia for his expertise and advice during the development of this protocol. The authors also wish to thank Mr. Razman Shah Mohd Razali, reference librarian, International Medical University for providing the full text articles whenever needed.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary 1 Search strategy for RCTs [Medline (via Ovid)]
The following search strategy will be used to identify RCTs of relevant interventions.
- Terms for colorectal cancer or adenomas
(exp Colorectal Neoplasms/ OR exp Colonic Neoplasms/ OR exp Rectal Neoplasms/ OR exp Adenomatous Polyps/ OR exp Adenocarcinoma/ OR exp Intestinal Polyps/ OR exp Colonic Polyps/) OR ((colorectal cancer$.tw OR colorectal tumo$.tw OR colorectal neoplas$.tw OR colon cancer$.tw OR colon tumo$.tw OR colon neoplas$.tw OR colonic cancer$.tw OR colonic tumo$.tw OR colonic neoplas$.tw OR rectal cancer$.tw OR rectal tumo$.tw OR rectal neoplas$.tw OR rectum cancer$.tw OR rectum tumo$.tw OR rectum neoplas$.tw OR polyp$.tw OR adenoma$.tw OR adenomatous$.tw) OR (exp Adenoma/)) - Terms for NSAIDs and aspirin
(exp Anti-Inflammatory Agents, Non-Steroidal/ OR exp cyclooxygenase inhibitors/ OR exp cyclooxygenase 2 inhibitors/ OR exp Aspirin/) OR (NSAID$.tw. OR Non-steroidal anti-inflammatory$.tw. OR Nonsteroidal anti-inflammatory$.tw. OR Non-steroidal antiinflammatory$.tw. OR Nonsteroidal antiinflammatory$.tw. OR Cyclo-oxygenase inhibitor$.tw. OR Cyclooxygenase inhibitor$.tw. OR Cyclooxygenase 1 inhibitor$.tw. OR Cyclooxygenase 2 inhibitor$.tw. OR COX-2 inhibitor$.tw. OR COX-2 selective inhibitor$.tw. OR COX-1 inhibitor$.tw. OR Coxib$.tw. OR Aspirin.af. OR Acetylsalicylic acid.tw.) - Terms for folic acid
exp Folic Acid/ OR folate$.tw. OR folic$.tw. OR folic Acid.af.) - Terms for calcium
(exp Calcium, Dietary/ OR exp Calcium/ OR calcium.tw.) - Terms for vitamin D
(exp Cholecalciferol/ OR exp Ergocalciferols/ OR vitamin d.tw. OR Cholecalciferol$.tw. OR Ergocalciferol$.tw.) - Terms for antioxidants
(exp Antioxidants/ OR anti-oxidant$.tw. OR antioxidant$.tw. OR Selenium/ OR exp Vitamin A/ OR exp Carotenoids/ OR carotenoid$.tw. OR beta-carotene.tw. OR exp Ascorbic Acid/ OR vitamin c.tw. OR exp Vitamin E/ OR exp Tocopherols/ OR Tocotrienols/ OR alpha-tocopherol$.tw. OR tocopherol$.tw. OR tocotrienol$.tw.) - Terms for randomized controlled trial
(randomized controlled trial.pt. OR controlled clinical trial.pt. OR exp Clinical Trial/ OR Randomized controlled trials/ OR random allocation/ OR double blind method/ OR single blind method/ OR clinical trial.pt. OR placebos/ OR placebo$.ti,ab. OR random$.tw OR blind$.ti,ab.) - (1 AND (2 OR 3 OR 4 OR 5 OR 6) AND 7)
- Limit 8 to (humans and yr=“2008 - 2015”)
Supplementary 2 Checklist of eligibility
The study will be excluded when there is a negative (“No”) answer to any of following questions:
- Is the study a randomized controlled trial?
- Yes ____
- No ____
- Unclear ____
- Are the participants comes under the following risk group?
- Average risk individuals: Individuals with no increased risk for colorectal cancer
- Increased risk individuals: Individuals at increased risk of colorectal cancer because of history of adenomatous polyps, personal history of colorectal cancer, family history of colorectal cancer, inflammatory bowel diseases
- High risk individuals: Individuals at high risk of colorectal cancer because of familial adenomatous polyposis (FAP), Lynch syndrome or hereditary nonpolyposis colorectal cancer (HNPCC)
- Yes ____
- No ____
- Unclear ____
- Is the intervention under treatment with any one of selected chemopreventive agents listed below?
Aspirin, non-aspirin NSAIDs (including COX-2 inhibitors), folic acid, calcium and/or vitamin D, antioxidants: vitamin A, vitamin C, vitamin E, selenium and beta-carotene. - Yes ____
- No ____
- Unclear ____
- Do the outcome measures include at least one of Incidence of colorectal cancer or Incidence/recurrence of any adenoma or change in polyp burden, number and size?
- Yes ____
- No ____
- Unclear ____
- Is the follow-up period at least 1 year?
- Yes ____
- No ____
- Unclear ____
- Is the study a non-duplicated and non-redundant publication?
- Yes ____
- No ____
- Unclear ____
Supplementary 3 Data extraction form
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Rustgi AK. The genetics of hereditary colon cancer. Genes Dev 2007;21:2525-38. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130-60. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Colorectal cancer screening rates remain low. [Internet]. 2013. Available online: http://www.cdc.gov/media/releases/2013/p1105-colorectal-cancer-screening.html
- Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med 2000;342:1960-8. [Crossref] [PubMed]
- Gloor FJ. The adenoma-carcinoma sequence of the colon and rectum. Soz Praventivmed 1986;31:74-5. [Crossref] [PubMed]
- Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251-70. [Crossref] [PubMed]
- Kim TI. Chemopreventive drugs: mechanisms via inhibition of cancer stem cells in colorectal cancer. World J Gastroenterol 2014;20:3835-46. [Crossref] [PubMed]
- Pan MH, Lai CS, Wu JC, et al. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res 2011;55:32-45. [Crossref] [PubMed]
- Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and Aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2004.CD004079. [PubMed]
- Cooper K, Squires H, Carroll C, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2010;14:1-206. [Crossref] [PubMed]
- Gao F, Liao C, Liu L, et al. The effect of aspirin in the recurrence of colorectal adenomas: a meta-analysis of randomized controlled trials. Colorectal Dis 2009;11:893-901. [Crossref] [PubMed]
- Rostom A, Dubé C, Lewin G, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 2007;146:376-89. [Crossref] [PubMed]
- Martínez ME, Willett WC. Calcium, vitamin D, and colorectal cancer: a review of the epidemiologic evidence. Cancer Epidemiol Biomarkers Prev 1998;7:163-8. [PubMed]
- Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res 1993;290:87-95. [Crossref] [PubMed]
- Le Marchand L, White KK, Nomura AM, et al. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:2195-201. [Crossref] [PubMed]
- Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr 2002;132:2350S-2355S. [PubMed]
- Sanjoaquin MA, Allen N, Couto E, et al. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005;113:825-8. [Crossref] [PubMed]
- Pais R, Dumitraşcu DL. Do antioxidants prevent colorectal cancer? A meta-analysis. Rom J Intern Med 2013;51:152-63. [PubMed]
- Hughes DJ, Fedirko V, Jenab M, et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer 2015;136:1149-61. [Crossref] [PubMed]
- Rudolf E, Králová V, Cervinka M. Selenium and colon cancer--from chemoprevention to new treatment modality. Anticancer Agents Med Chem 2008;8:598-602. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;146:361-4. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Final recommendation statement: Aspirin/NSAIDs for prevention of colorectal cancer: preventive medication. U.S. Preventive Services Task Force, February 2014. Available online: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-nsaids-for-prevention-of-colorectal-cancer-preventive-medication
- Arain MA, Abdul Qadeer A. Systematic review on "vitamin E and prevention of colorectal cancer". Pak J Pharm Sci 2010;23:125-30. [PubMed]
- Bjelakovic G, Nagorni A, Nikolova D, et al. Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma. Aliment Pharmacol Ther 2006;24:281-91. [Crossref] [PubMed]
- Brotons C, Benamouzig R, Filipiak KJ, et al. A systematic review of aspirin in primary prevention: is it time for a new approach? Am J Cardiovasc Drugs 2015;15:113-33. [Crossref] [PubMed]
- Carroll C, Cooper K, Papaioannou D, et al. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther 2010;31:708-18. [Crossref] [PubMed]
- Carroll C, Cooper K, Papaioannou D, et al. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther 2010;32:789-803. [Crossref] [PubMed]
- Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 2009;101:256-66. [Crossref] [PubMed]
- Figueiredo JC, Mott LA, Giovannucci E, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer 2011;129:192-203. [Crossref] [PubMed]
- Heine-Bröring RC, Winkels RM, Renkema JM, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer 2015;136:2388-401. [Crossref] [PubMed]
- Kennedy DA, Stern SJ, Moretti M, et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol 2011;35:2-10. [Crossref] [PubMed]
- Ma Y, Zhang P, Wang F, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 2011;29:3775-82. [Crossref] [PubMed]
- Papaioannou D, Cooper KL, Carroll C, et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Dis 2011;13:1085-99. [Crossref] [PubMed]
- Qin X, Cui Y, Shen L, et al. Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials. Int J Cancer 2013;133:1033-41. [Crossref] [PubMed]
- Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012;379:1591-601. [Crossref] [PubMed]
- Shaukat A, Scouras N, Schünemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol 2005;100:390-4. [Crossref] [PubMed]
- Wang YP, Wang Q, Gan T, et al. Non-steroidal anti-inflammatory agents for chemoprevention of colorectal polyps: a meta-analysis. Zhonghua Nei Ke Za Zhi 2004;43:10-2. [PubMed]
- Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev 2005.CD003548. [PubMed]
- Wien TN, Pike E, Wisløff T, et al. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open 2012;2:e000653. [Crossref] [PubMed]
- Ye X, Fu J, Yang Y, et al. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PLoS One 2013;8:e57578. [Crossref] [PubMed]
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health 2011;14:429-37. [Crossref] [PubMed]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. [Crossref] [PubMed]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [Crossref] [PubMed]
- Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc 2006;63:546-57. [Crossref] [PubMed]
- Sun SX, Lee KY, Bertram CT, et al. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin 2007;23:1859-66. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. Available online: http://handbook.cochrane.org/
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332-45. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [Crossref] [PubMed]
- Veettil SK, Chaiyakunapruk N, Saokaew S, et al. Comparative effectiveness of chemopreventive interventions for colorectal cancer: protocol for a systematic review and network meta-analysis. PROSPERO 2015:CRD42015025849. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025849